MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles
- PMID: 22911431
- PMCID: PMC3406102
- DOI: 10.1371/journal.ppat.1002829
MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious hepatitis C virus particles
Abstract
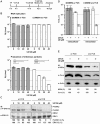
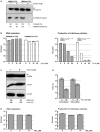
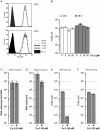
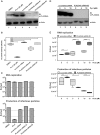
Hepatitis C virus (HCV) has infected around 160 million individuals. Current therapies have limited efficacy and are fraught with side effects. To identify cellular HCV dependency factors, possible therapeutic targets, we manipulated signaling cascades with pathway-specific inhibitors. Using this approach we identified the MAPK/ERK regulated, cytosolic, calcium-dependent, group IVA phospholipase A2 (PLA2G4A) as a novel HCV dependency factor. Inhibition of PLA2G4A activity reduced core protein abundance at lipid droplets, core envelopment and secretion of particles. Moreover, released particles displayed aberrant protein composition and were 100-fold less infectious. Exogenous addition of arachidonic acid, the cleavage product of PLA2G4A-catalyzed lipolysis, but not other related poly-unsaturated fatty acids restored infectivity. Strikingly, production of infectious Dengue virus, a relative of HCV, was also dependent on PLA2G4A. These results highlight previously unrecognized parallels in the assembly pathways of these human pathogens, and define PLA2G4A-dependent lipolysis as crucial prerequisite for production of highly infectious viral progeny.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Cytosolic phospholipase A2 gamma is involved in hepatitis C virus replication and assembly.J Virol. 2012 Dec;86(23):13025-37. doi: 10.1128/JVI.01785-12. Epub 2012 Sep 26. J Virol. 2012. PMID: 23015700 Free PMC article.
-
Involvement of ERK pathway in interferon alpha-mediated antiviral activity against hepatitis C virus.Cytokine. 2015 Mar;72(1):17-24. doi: 10.1016/j.cyto.2014.11.031. Epub 2014 Dec 23. Cytokine. 2015. PMID: 25544181
-
Oxidative stress induces anti-hepatitis C virus status via the activation of extracellular signal-regulated kinase.Hepatology. 2009 Sep;50(3):678-88. doi: 10.1002/hep.23026. Hepatology. 2009. PMID: 19492433
-
Nonstructural 3 Protein of Hepatitis C Virus Modulates the Tribbles Homolog 3/Akt Signaling Pathway for Persistent Viral Infection.J Virol. 2016 Jul 27;90(16):7231-7247. doi: 10.1128/JVI.00326-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252525 Free PMC article.
-
Signal transduction pathways for activation of extracellular signal-regulated kinase by arachidonic acid in rat neutrophils.J Leukoc Biol. 2001 Apr;69(4):659-65. J Leukoc Biol. 2001. PMID: 11310854
Cited by
-
Whole Lotta Lipids-from HCV RNA Replication to the Mature Viral Particle.Int J Mol Sci. 2020 Apr 21;21(8):2888. doi: 10.3390/ijms21082888. Int J Mol Sci. 2020. PMID: 32326151 Free PMC article. Review.
-
The ins and outs of hepatitis C virus entry and assembly.Nat Rev Microbiol. 2013 Oct;11(10):688-700. doi: 10.1038/nrmicro3098. Epub 2013 Sep 10. Nat Rev Microbiol. 2013. PMID: 24018384 Free PMC article. Review.
-
Hepatitis C virus RNA replication and assembly: living on the fat of the land.Cell Host Microbe. 2014 Nov 12;16(5):569-79. doi: 10.1016/j.chom.2014.10.008. Epub 2014 Nov 12. Cell Host Microbe. 2014. PMID: 25525790 Free PMC article. Review.
-
Interplay between Hepatitis C Virus and Redox Cell Signaling.Int J Mol Sci. 2013 Feb 26;14(3):4705-21. doi: 10.3390/ijms14034705. Int J Mol Sci. 2013. PMID: 23443167 Free PMC article.
-
The Role of Noncoding RNA in the Transmission and Pathogenicity of Flaviviruses.Viruses. 2024 Feb 2;16(2):242. doi: 10.3390/v16020242. Viruses. 2024. PMID: 38400018 Free PMC article. Review.
References
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. - PubMed
-
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:521, e511–516. - PubMed
-
- Lohmann V, Korner F, Koch J, Herian U, Theilmann L, et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

