Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway
- PMID: 22910909
- PMCID: PMC3471719
- DOI: 10.1074/jbc.M112.348300
Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway
Abstract
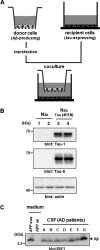
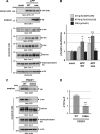
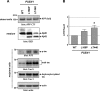
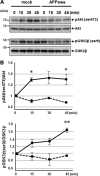
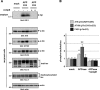
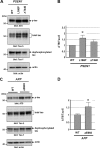
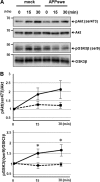
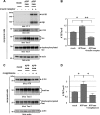
Alzheimer disease (AD) is neuropathologically characterized by the formation of senile plaques from amyloid-β (Aβ) and neurofibrillary tangles composed of phosphorylated Tau. Although there is growing evidence for the pathogenic role of soluble Aβ species in AD, the major question of how Aβ induces hyperphosphorylation of Tau remains unanswered. To address this question, we here developed a novel cell coculture system to assess the effect of extracellular Aβ at physiologically relevant levels naturally secreted from donor cells on the phosphorylation of Tau in recipient cells. Using this assay, we demonstrated that physiologically relevant levels of secreted Aβ are sufficient to cause hyperphosphorylation of Tau in recipient N2a cells expressing human Tau and in primary culture neurons. This hyperphosphorylation of Tau is inhibited by blocking Aβ production in donor cells. The expression of familial AD-linked PSEN1 mutants and APP ΔE693 mutant that induce the production of oligomeric Aβ in donor cells results in a similar hyperphosphorylation of Tau in recipient cells. The mechanism underlying the Aβ-induced Tau hyperphosphorylation is mediated by the impaired insulin signal transduction because we demonstrated that the phosphorylation of Akt and GSK3β upon insulin stimulation is less activated under this condition. Treating cells with the insulin-sensitizing drug rosiglitazone, a peroxisome proliferator-activated receptor γ agonist, attenuates the Aβ-dependent hyperphosphorylation of Tau. These findings suggest that the disturbed insulin signaling cascade may be implicated in the pathways through which soluble Aβ induces Tau phosphorylation and further support the notion that correcting insulin signal dysregulation in AD may offer a potential therapeutic approach.
Figures

Similar articles
-
FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer's disease via decreasing beta-amyloid production and tau hyperphosphorylation.PLoS One. 2013 Nov 4;8(11):e78033. doi: 10.1371/journal.pone.0078033. eCollection 2013. PLoS One. 2013. PMID: 24223757 Free PMC article.
-
Age-dependent accumulation of soluble amyloid beta (Abeta) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPP(alpha)) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer mouse model.J Biol Chem. 2011 May 27;286(21):18414-25. doi: 10.1074/jbc.M110.209718. Epub 2011 Apr 1. J Biol Chem. 2011. PMID: 21460223 Free PMC article.
-
The spleen tyrosine kinase (Syk) regulates Alzheimer amyloid-β production and Tau hyperphosphorylation.J Biol Chem. 2014 Dec 5;289(49):33927-44. doi: 10.1074/jbc.M114.608091. Epub 2014 Oct 20. J Biol Chem. 2014. PMID: 25331948 Free PMC article.
-
Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Tau and neuron aging.Aging Dis. 2013 Feb;4(1):23-8. Epub 2012 Dec 3. Aging Dis. 2013. PMID: 23423462 Free PMC article.
-
Study on Mechanism of Ginkgo biloba L. Leaves for the Treatment of Neurodegenerative Diseases Based on Network Pharmacology.Neurochem Res. 2021 Jul;46(7):1881-1894. doi: 10.1007/s11064-021-03315-z. Epub 2021 May 14. Neurochem Res. 2021. PMID: 33988813
-
Extracellular Vesicle Biomarkers Track Cognitive Changes Following Intranasal Insulin in Alzheimer's Disease.J Alzheimers Dis. 2019;69(2):489-498. doi: 10.3233/JAD-180578. J Alzheimers Dis. 2019. PMID: 30958348 Free PMC article. Clinical Trial.
-
The Neuroprotective Effects of Justicidin A on Amyloid Beta25-35-Induced Neuronal Cell Death Through Inhibition of Tau Hyperphosphorylation and Induction of Autophagy in SH-SY5Y Cells.Neurochem Res. 2016 Jun;41(6):1458-67. doi: 10.1007/s11064-016-1857-5. Epub 2016 Feb 18. Neurochem Res. 2016. PMID: 26887582
-
Rise and fall of peroxisomes during Alzheimer´s disease: a pilot study in human brains.Acta Neuropathol Commun. 2023 May 11;11(1):80. doi: 10.1186/s40478-023-01567-0. Acta Neuropathol Commun. 2023. PMID: 37170361 Free PMC article.
References
-
- Gong Y., Chang L., Viola K. L., Lacor P. N., Lambert M. P., Finch C. E., Krafft G. A., Klein W. L. (2003) Alzheimer's disease-affected brain. Presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U.S.A. 100, 10417–10422 - PMC - PubMed
-
- Klyubin I., Walsh D. M., Lemere C. A., Cullen W. K., Shankar G. M., Betts V., Spooner E. T., Jiang L., Anwyl R., Selkoe D. J., Rowan M. J. (2005) Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 11, 556–561 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

