Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2
- PMID: 22908275
- PMCID: PMC3437859
- DOI: 10.1073/pnas.1203201109
Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2
Abstract
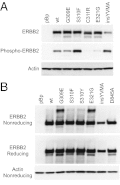
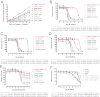
We assessed somatic alleles of six receptor tyrosine kinase genes mutated in lung adenocarcinoma for oncogenic activity. Five of these genes failed to score in transformation assays; however, novel recurring extracellular domain mutations of the receptor tyrosine kinase gene ERBB2 were potently oncogenic. These ERBB2 extracellular domain mutants were activated by two distinct mechanisms, characterized by elevated C-terminal tail phosphorylation or by covalent dimerization mediated by intermolecular disulfide bond formation. These distinct mechanisms of receptor activation converged upon tyrosine phosphorylation of cellular proteins, impacting cell motility. Survival of Ba/F3 cells transformed to IL-3 independence by the ERBB2 extracellular domain mutants was abrogated by treatment with small-molecule inhibitors of ERBB2, raising the possibility that patients harboring such mutations could benefit from ERBB2-directed therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mutational landscape and characteristics of ERBB2 in non-small cell lung cancer.Thorac Cancer. 2020 Jun;11(6):1512-1521. doi: 10.1111/1759-7714.13419. Epub 2020 Apr 14. Thorac Cancer. 2020. PMID: 32291971 Free PMC article.
-
Allele-Specific Role of ERBB2 in the Oncogenic Function of EGFR L861Q in EGFR-Mutant Lung Cancers.J Thorac Oncol. 2021 Jan;16(1):113-126. doi: 10.1016/j.jtho.2020.09.019. Epub 2020 Oct 7. J Thorac Oncol. 2021. PMID: 33038514 Free PMC article.
-
Lung cancer: intragenic ERBB2 kinase mutations in tumours.Nature. 2004 Sep 30;431(7008):525-6. doi: 10.1038/431525b. Nature. 2004. PMID: 15457249
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
-
The ErbB/HER receptor protein-tyrosine kinases and cancer.Biochem Biophys Res Commun. 2004 Jun 18;319(1):1-11. doi: 10.1016/j.bbrc.2004.04.150. Biochem Biophys Res Commun. 2004. PMID: 15158434 Review.
Cited by
-
Loss of progesterone receptor is associated with distinct tyrosine kinase profiles in breast cancer.Breast Cancer Res Treat. 2020 Oct;183(3):585-598. doi: 10.1007/s10549-020-05763-7. Epub 2020 Jul 24. Breast Cancer Res Treat. 2020. PMID: 32710281 Free PMC article.
-
HER2 activating mutations are targets for colorectal cancer treatment.Cancer Discov. 2015 Aug;5(8):832-41. doi: 10.1158/2159-8290.CD-14-1211. Cancer Discov. 2015. PMID: 26243863 Free PMC article.
-
Corrigendum: Comprehensive genomic characterization defines human glioblastoma genes and core pathways.Nature. 2013 Feb 6;494(7438):506. doi: 10.1038/nature11903. Epub 2013 Feb 6. Nature. 2013. PMID: 23389443 No abstract available.
-
A neu view of invasive lobular breast cancer.Clin Cancer Res. 2013 Jul 1;19(13):3331-3. doi: 10.1158/1078-0432.CCR-13-1031. Epub 2013 May 28. Clin Cancer Res. 2013. PMID: 23714731 Free PMC article.
-
Identification of an Activating Mutation in the Extracellular Domain of HER2 Conferring Resistance to Pertuzumab.Onco Targets Ther. 2019 Dec 30;12:11597-11608. doi: 10.2147/OTT.S232912. eCollection 2019. Onco Targets Ther. 2019. PMID: 31920346 Free PMC article.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. - PubMed
-
- Sharma SV, Settleman J. Exploiting the balance between life and death: Targeted cancer therapy and “oncogenic shock”. Biochem Pharmacol. 2010;80:666–673. - PubMed
-
- Weinstein IB. Cancer. Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–64. - PubMed
-
- Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. - PubMed
-
- Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

