Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation
- PMID: 22908252
- PMCID: PMC3437889
- DOI: 10.1073/pnas.1208228109
Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation
Abstract
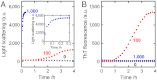
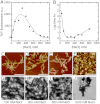
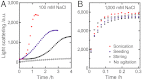
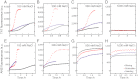
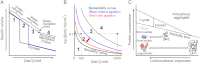
Amyloid fibrils and amorphous aggregates are two types of aberrant aggregates associated with protein misfolding diseases. Although they differ in morphology, the two forms are often treated indiscriminately. β(2)-microglobulin (β2m), a protein responsible for dialysis-related amyloidosis, forms amyloid fibrils or amorphous aggregates depending on the NaCl concentration at pH 2.5. We compared the kinetics of their formation, which was monitored by measuring thioflavin T fluorescence, light scattering, and 8-anilino-1-naphthalenesulfonate fluorescence. Thioflavin T fluorescence specifically monitors amyloid fibrillation, whereas light scattering and 8-anilino-1-naphthalenesulfonate fluorescence monitor both amyloid fibrillation and amorphous aggregation. The amyloid fibrils formed via a nucleation-dependent mechanism in a supersaturated solution, analogous to crystallization. The lag phase of fibrillation was reduced upon agitation with stirring or ultrasonic irradiation, and disappeared by seeding with preformed fibrils. In contrast, the glass-like amorphous aggregates formed rapidly without a lag phase. Neither agitation nor seeding accelerated the amorphous aggregation. Thus, by monitoring the kinetics, we can distinguish between crystal-like amyloid fibrils and glass-like amorphous aggregates. Solubility and supersaturation will be key factors for further understanding the aberrant aggregation of proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aggregation-phase diagrams of β2-microglobulin reveal temperature and salt effects on competitive formation of amyloids versus amorphous aggregates.J Biol Chem. 2018 Sep 21;293(38):14775-14785. doi: 10.1074/jbc.RA118.004683. Epub 2018 Aug 3. J Biol Chem. 2018. PMID: 30077972 Free PMC article.
-
Supersaturation-limited and Unlimited Phase Transitions Compete to Produce the Pathway Complexity in Amyloid Fibrillation.J Biol Chem. 2015 Jul 17;290(29):18134-18145. doi: 10.1074/jbc.M115.648139. Epub 2015 Jun 10. J Biol Chem. 2015. PMID: 26063798 Free PMC article.
-
Heating during agitation of β2-microglobulin reveals that supersaturation breakdown is required for amyloid fibril formation at neutral pH.J Biol Chem. 2019 Oct 25;294(43):15826-15835. doi: 10.1074/jbc.RA119.009971. Epub 2019 Sep 8. J Biol Chem. 2019. PMID: 31495783 Free PMC article.
-
Supersaturation, a Critical Factor Underlying Proteostasis of Amyloid Fibril Formation.J Mol Biol. 2024 Jul 15;436(14):168475. doi: 10.1016/j.jmb.2024.168475. Epub 2024 Feb 3. J Mol Biol. 2024. PMID: 38311232 Review.
-
Supersaturation-Dependent Formation of Amyloid Fibrils.Molecules. 2022 Jul 19;27(14):4588. doi: 10.3390/molecules27144588. Molecules. 2022. PMID: 35889461 Free PMC article. Review.
Cited by
-
Selective recognition and discrimination of single isomeric changes in peptide strands with a host : guest sensing array.Chem Sci. 2024 Jan 2;15(5):1885-1893. doi: 10.1039/d3sc06087j. eCollection 2024 Jan 31. Chem Sci. 2024. PMID: 38303931 Free PMC article.
-
Relevance of Amorphous and Amyloid-Like Aggregates of the p53 Core Domain to Loss of its DNA-Binding Activity.Front Mol Biosci. 2022 Apr 26;9:869851. doi: 10.3389/fmolb.2022.869851. eCollection 2022. Front Mol Biosci. 2022. PMID: 35558561 Free PMC article.
-
Distribution and Structure Analysis of Fibril-Forming Peptides Focusing on Concentration Dependency.ACS Omega. 2022 Mar 14;7(12):10012-10021. doi: 10.1021/acsomega.1c04960. eCollection 2022 Mar 29. ACS Omega. 2022. PMID: 35382341 Free PMC article.
-
A cationic polymethacrylate-copolymer acts as an agonist for β-amyloid and an antagonist for amylin fibrillation.Chem Sci. 2019 Feb 27;10(14):3976-3986. doi: 10.1039/c8sc05771k. eCollection 2019 Apr 14. Chem Sci. 2019. PMID: 31015938 Free PMC article.
-
A specific form of prefibrillar aggregates that functions as a precursor of amyloid nucleation.Sci Rep. 2018 Jan 8;8(1):62. doi: 10.1038/s41598-017-18390-y. Sci Rep. 2018. PMID: 29311640 Free PMC article.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Sugiyama M, et al. SAXS and SANS observations of abnormal aggregation of human α-crystallin. Chem Biodiversity. 2010;7:1380–1388. - PubMed
-
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. - PubMed
-
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

