Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes
- PMID: 22901542
- PMCID: PMC3864037
- DOI: 10.1016/j.chom.2012.06.006
Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes
Abstract
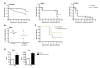
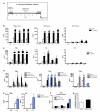
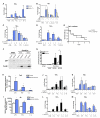
Immunological memory in vertebrates is often exclusively attributed to T and B cell function. Recently it was proposed that the enhanced and sustained innate immune responses following initial infectious exposure may also afford protection against reinfection. Testing this concept of "trained immunity," we show that mice lacking functional T and B lymphocytes are protected against reinfection with Candida albicans in a monocyte-dependent manner. C. albicans and fungal cell wall β-glucans induced functional reprogramming of monocytes, leading to enhanced cytokine production in vivo and in vitro. The training required the β-glucan receptor dectin-1 and the noncanonical Raf-1 pathway. Monocyte training by β-glucans was associated with stable changes in histone trimethylation at H3K4, which suggests the involvement of epigenetic mechanisms in this phenomenon. The functional reprogramming of monocytes, reminiscent of similar NK cell properties, supports the concept of "trained immunity" and may be employed for the design of improved vaccination strategies.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Phagocytes from Mice Lacking the Sts Phosphatases Have an Enhanced Antifungal Response to Candida albicans.mBio. 2018 Jul 17;9(4):e00782-18. doi: 10.1128/mBio.00782-18. mBio. 2018. PMID: 30018105 Free PMC article.
-
The protective effect of inflammatory monocytes during systemic C. albicans infection is dependent on collaboration between C-type lectin-like receptors.PLoS Pathog. 2019 Jun 26;15(6):e1007850. doi: 10.1371/journal.ppat.1007850. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31242262 Free PMC article.
-
Dectin-1 Stimulation of Hematopoietic Stem and Progenitor Cells Occurs In Vivo and Promotes Differentiation Toward Trained Macrophages via an Indirect Cell-Autonomous Mechanism.mBio. 2020 Jun 23;11(3):e00781-20. doi: 10.1128/mBio.00781-20. mBio. 2020. PMID: 32576672 Free PMC article.
-
CARD9 Syk-dependent and Raf-1 Syk-independent signaling pathways in target recognition of Candida albicans by Dectin-1.Eur J Clin Microbiol Infect Dis. 2011 Mar;30(3):303-5. doi: 10.1007/s10096-010-1103-z. Epub 2010 Nov 25. Eur J Clin Microbiol Infect Dis. 2011. PMID: 21108038 Review.
-
Trained immunity: A smart way to enhance innate immune defence.Mol Immunol. 2015 Nov;68(1):40-4. doi: 10.1016/j.molimm.2015.06.019. Mol Immunol. 2015. PMID: 26597205 Review.
Cited by
-
The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity.EMBO Rep. 2024 Dec;25(12):5239-5264. doi: 10.1038/s44319-024-00296-2. Epub 2024 Oct 31. EMBO Rep. 2024. PMID: 39482490 Free PMC article. Review.
-
The roles of inducible chromatin and transcriptional memory in cellular defense system responses to redox-active pollutants.Free Radic Biol Med. 2021 Jul;170:85-108. doi: 10.1016/j.freeradbiomed.2021.03.018. Epub 2021 Mar 28. Free Radic Biol Med. 2021. PMID: 33789123 Free PMC article. Review.
-
BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner.J Clin Invest. 2020 Oct 1;130(10):5591-5602. doi: 10.1172/JCI133935. J Clin Invest. 2020. PMID: 32692728 Free PMC article.
-
Vaccination with O-linked Mannans Protects against Systemic Candidiasis through Innate Lymphocyte Populations.J Immunol. 2024 Sep 15;213(6):843-852. doi: 10.4049/jimmunol.2400065. J Immunol. 2024. PMID: 39109925
-
Immunogenetics associated with severe coccidioidomycosis.JCI Insight. 2022 Nov 22;7(22):e159491. doi: 10.1172/jci.insight.159491. JCI Insight. 2022. PMID: 36166305 Free PMC article.
References
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
-
- Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes Infect. 2007;9:1680–1687. - PubMed
-
- Brown GD, Williams DL. (1,3)-β-Glucans in Innate Immunity: Mammalian Systems. In: Bacic A, Fincher GB, Stone BA, editors. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides. Academic Press; Amsterdam: 2009. pp. 579–619.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
