Redox regulation of sodium and calcium handling
- PMID: 22900788
- PMCID: PMC3567778
- DOI: 10.1089/ars.2012.4818
Redox regulation of sodium and calcium handling
Abstract
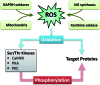
Significance: In heart failure (HF), contractile dysfunction and arrhythmias result from disturbed intracellular Ca handling. Activated stress kinases like cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), and Ca/calmodulin-dependent protein kinase II (CaMKII), which are known to influence many Ca-regulatory proteins, are mechanistically involved.
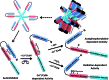
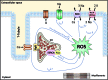
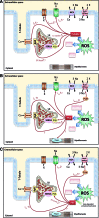
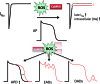
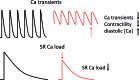
Recent advances: Beside classical activation pathways, it is becoming increasingly evident that reactive oxygen species (ROS) can directly oxidize these kinases, leading to alternative activation. Since HF is associated with increased ROS generation, ROS-activated serine/threonine kinases may play a crucial role in the disturbance of cellular Ca homeostasis. Many of the previously described ROS effects on ion channels and transporters are possibly mediated by these stress kinases. For instance, ROS have been shown to oxidize and activate CaMKII, thereby increasing Na influx through voltage-gated Na channels, which can lead to intracellular Na accumulation and action potential prolongation. Consequently, Ca entry via activated NCX is favored, which together with ROS-induced dysfunction of the sarcoplasmic reticulum can lead to dramatic intracellular Ca accumulation, diminished contractility, and arrhythmias.
Critical issues: While low amounts of ROS may regulate kinase activity, excessive uncontrolled ROS production may lead to direct redox modification of Ca handling proteins. Therefore, depending on the source and amount of ROS generated, ROS could have very different effects on Ca-handling proteins.
Future directions: The discrimination between fine-tuned ROS signaling and unspecific ROS damage may be crucial for the understanding of heart failure development and important for the investigation of targeted treatment strategies.
Figures
Similar articles
-
NADPH oxidase 2 mediates angiotensin II-dependent cellular arrhythmias via PKA and CaMKII.J Mol Cell Cardiol. 2014 Oct;75:206-15. doi: 10.1016/j.yjmcc.2014.07.011. Epub 2014 Jul 27. J Mol Cell Cardiol. 2014. PMID: 25073061
-
Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes.Free Radic Biol Med. 2013 Oct;63:338-49. doi: 10.1016/j.freeradbiomed.2013.05.035. Epub 2013 Jun 1. Free Radic Biol Med. 2013. PMID: 23732518 Review.
-
Mechanistic Investigation of the Arrhythmogenic Role of Oxidized CaMKII in the Heart.Biophys J. 2015 Aug 18;109(4):838-49. doi: 10.1016/j.bpj.2015.06.064. Biophys J. 2015. PMID: 26287635 Free PMC article.
-
Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload.Circ Res. 2011 Mar 4;108(5):555-65. doi: 10.1161/CIRCRESAHA.110.221911. Epub 2011 Jan 20. Circ Res. 2011. PMID: 21252154 Free PMC article.
-
Calcium Signaling and Reactive Oxygen Species in Mitochondria.Circ Res. 2018 May 11;122(10):1460-1478. doi: 10.1161/CIRCRESAHA.118.310082. Circ Res. 2018. PMID: 29748369 Review.
Cited by
-
Arrhythmogenic Mechanisms in Heart Failure: Linking β-Adrenergic Stimulation, Stretch, and Calcium.Front Physiol. 2018 Oct 16;9:1453. doi: 10.3389/fphys.2018.01453. eCollection 2018. Front Physiol. 2018. PMID: 30374311 Free PMC article. Review.
-
Intermittent Hypoxia Prevents Myocardial Mitochondrial Ca2+ Overload and Cell Death during Ischemia/Reperfusion: The Role of Reactive Oxygen Species.Cells. 2019 Jun 9;8(6):564. doi: 10.3390/cells8060564. Cells. 2019. PMID: 31181855 Free PMC article.
-
Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle.Antioxid Redox Signal. 2014 Feb 20;20(6):929-36. doi: 10.1089/ars.2013.5517. Epub 2014 Jan 3. Antioxid Redox Signal. 2014. PMID: 23971496 Free PMC article. Review.
-
p21-Activated kinase1 (Pak1) is a negative regulator of NADPH-oxidase 2 in ventricular myocytes.J Mol Cell Cardiol. 2014 Feb;67:77-85. doi: 10.1016/j.yjmcc.2013.12.017. Epub 2013 Dec 28. J Mol Cell Cardiol. 2014. PMID: 24380729 Free PMC article.
-
ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements.J Clin Med. 2020 May 2;9(5):1328. doi: 10.3390/jcm9051328. J Clin Med. 2020. PMID: 32370294 Free PMC article. Review.
References
-
- Abramson JJ. Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21:283–294. - PubMed
-
- Akki A. Zhang M. Murdoch C. Brewer A. Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. - PubMed
-
- Anilkumar N. Sirker A. Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

