Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate
- PMID: 22894855
- PMCID: PMC3501149
- DOI: 10.1021/cb3002478
Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate
Abstract
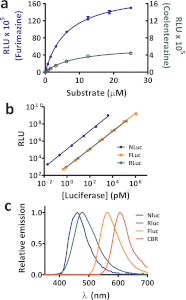
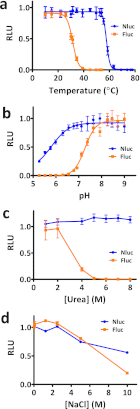
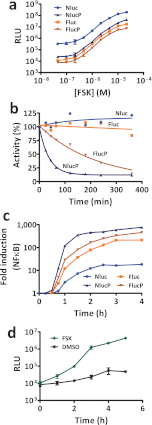
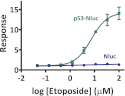
Bioluminescence methodologies have been extraordinarily useful due to their high sensitivity, broad dynamic range, and operational simplicity. These capabilities have been realized largely through incremental adaptations of native enzymes and substrates, originating from luminous organisms of diverse evolutionary lineages. We engineered both an enzyme and substrate in combination to create a novel bioluminescence system capable of more efficient light emission with superior biochemical and physical characteristics. Using a small luciferase subunit (19 kDa) from the deep sea shrimp Oplophorus gracilirostris, we have improved luminescence expression in mammalian cells ~2.5 million-fold by merging optimization of protein structure with development of a novel imidazopyrazinone substrate (furimazine). The new luciferase, NanoLuc, produces glow-type luminescence (signal half-life >2 h) with a specific activity ~150-fold greater than that of either firefly (Photinus pyralis) or Renilla luciferases similarly configured for glow-type assays. In mammalian cells, NanoLuc shows no evidence of post-translational modifications or subcellular partitioning. The enzyme exhibits high physical stability, retaining activity with incubation up to 55 °C or in culture medium for >15 h at 37 °C. As a genetic reporter, NanoLuc may be configured for high sensitivity or for response dynamics by appending a degradation sequence to reduce intracellular accumulation. Appending a signal sequence allows NanoLuc to be exported to the culture medium, where reporter expression can be measured without cell lysis. Fusion onto other proteins allows luminescent assays of their metabolism or localization within cells. Reporter quantitation is achievable even at very low expression levels to facilitate more reliable coupling with endogenous cellular processes.
Figures



Similar articles
-
Reporter enzyme inhibitor study to aid assembly of orthogonal reporter gene assays.ACS Chem Biol. 2013 May 17;8(5):1009-17. doi: 10.1021/cb3007264. Epub 2013 Mar 26. ACS Chem Biol. 2013. PMID: 23485150
-
Secretional luciferase of the luminous shrimp Oplophorus gracilirostris: cDNA cloning of a novel imidazopyrazinone luciferase(1).FEBS Lett. 2000 Sep 8;481(1):19-25. doi: 10.1016/s0014-5793(00)01963-3. FEBS Lett. 2000. PMID: 10984608
-
Optimization of enzyme-substrate pairing for bioluminescence imaging of gene transfer using Renilla and Gaussia luciferases.J Gene Med. 2010 Jun;12(6):528-37. doi: 10.1002/jgm.1463. J Gene Med. 2010. PMID: 20527045 Free PMC article.
-
Firefly Luciferase-based Fusion Proteins and their Applications in Bioanalysis.Photochem Photobiol. 2017 Mar;93(2):436-447. doi: 10.1111/php.12656. Epub 2016 Nov 30. Photochem Photobiol. 2017. PMID: 27796044 Review.
-
Imaging of light emission from the expression of luciferases in living cells and organisms: a review.Luminescence. 2002 Jan-Feb;17(1):43-74. doi: 10.1002/bio.676. Luminescence. 2002. PMID: 11816060 Review.
Cited by
-
Genome editing in mammalian cells using the CRISPR type I-D nuclease.Nucleic Acids Res. 2021 Jun 21;49(11):6347-6363. doi: 10.1093/nar/gkab348. Nucleic Acids Res. 2021. PMID: 34076237 Free PMC article.
-
Studies on Simultaneous Enrichment and Detection of Escherichia coli O157:H7 during Sample Shipment.Foods. 2022 Nov 15;11(22):3653. doi: 10.3390/foods11223653. Foods. 2022. PMID: 36429244 Free PMC article.
-
A Luminescence Assay to Quantify Cell Viability in Real Time.Methods Mol Biol. 2021;2255:187-196. doi: 10.1007/978-1-0716-1162-3_16. Methods Mol Biol. 2021. PMID: 34033104
-
Tools and techniques for illuminating the cell biology of zinc.Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118865. doi: 10.1016/j.bbamcr.2020.118865. Epub 2020 Sep 24. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 32980354 Free PMC article. Review.
-
Turning Antibodies into Ratiometric Bioluminescent Sensors for Competition-Based Homogeneous Immunoassays.ACS Sens. 2024 Mar 22;9(3):1401-1409. doi: 10.1021/acssensors.3c02478. Epub 2024 Feb 21. ACS Sens. 2024. PMID: 38380622 Free PMC article.
References
-
- Widder E. A. (2010) Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328, 704–708. - PubMed
-
- Melnick J. S.; Janes J.; Kim S.; Chang J. Y.; Sipes D. G.; Gunderson D.; Jarnes L.; Matzen J. T.; Garcia M. E.; Hood T. L.; Beigi R.; Xia G.; Harig R. A.; Asatryan H.; Yan S. F.; Zhou Y.; Gu X. J.; Saadat A.; Zhou V.; King F. J.; Shaw C. M.; Su A. I.; Downs R.; Gray N. S.; Schultz P. G.; Warmuth M.; Caldwell J. S. (2006) An efficient rapid system for profiling the cellular activities of molecular libraries. Proc. Natl. Acad. Sci. U.S.A. 103, 3153–3158. - PMC - PubMed
-
- Doshi U.; Li A. P. (2011) Luciferin IPA-based higher throughput human hepatocyte screening assays for CYP3A4 inhibition and induction. J. Biomol. Screen. 16, 903–909. - PubMed
-
- Smirnova N. A.; Haskew-Layton R. E.; Basso M.; Hushpulian D. M.; Payappilly J. B.; Speer R. E.; Ahn Y. H.; Rakhman I.; Cole P. A.; Pinto J. T.; Ratan R. R.; Gazaryan I. G. (2011) Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem. Biol. 18, 752–765. - PMC - PubMed
-
- Perroy J.; Pontier S.; Charest P. G.; Aubry M.; Bouvier M. (2004) Real-time monitoring of ubiquitination in living cells by BRET. Nat. Methods 1, 203–208. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

