Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules
- PMID: 22891853
- PMCID: PMC3542901
- DOI: 10.1089/ten.TEA.2011.0738
Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules
Abstract
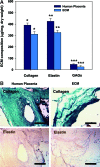
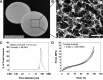
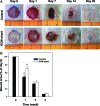
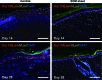
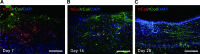
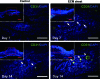
The human placenta, a complex organ, which facilitates exchange between the fetus and the mother, contains abundant extracellular matrix (ECM) components and well-preserved endogenous growth factors. In this study, we designed a new dermal substitute from human placentas for full-thickness wound healing. Highly porous, decellularized ECM sheets were fabricated from human placentas via homogenization, centrifugation, chemical and enzymatic treatments, molding, and freeze-drying. The physical structure and biological composition of human placenta-derived ECM sheets dramatically supported the regeneration of full-thickness wound in vivo. At the early stage, the ECM sheet efficiently absorbed wound exudates and tightly attached to the wound surface. Four weeks after implantation, the wound was completely closed, epidermic cells were well arranged and the bilayer structure of the epidermis and dermis was restored. Moreover, hair follicles and microvessels were newly formed in the ECM sheet-implanted wounds. Overall, the ECM sheet produced a dermal substitute with similar cellular organization to that of normal skin. These results suggest that human placenta-derived ECM sheets provide a microenvironment favorable to the growth and differentiation of cells, and positive modulate the healing of full-thickness wounds.
Figures



Similar articles
-
Wound-healing effect of adipose stem cell-derived extracellular matrix sheet on full-thickness skin defect rat model: Histological and immunohistochemical study.Int Wound J. 2019 Feb;16(1):286-296. doi: 10.1111/iwj.13030. Epub 2018 Nov 20. Int Wound J. 2019. PMID: 30461211 Free PMC article.
-
Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration.ACS Appl Mater Interfaces. 2018 May 23;10(20):16977-16991. doi: 10.1021/acsami.7b19007. Epub 2018 May 11. ACS Appl Mater Interfaces. 2018. PMID: 29718653
-
Growth Factor-Reinforced ECM Fabricated from Chemically Hypoxic MSC Sheet with Improved In Vivo Wound Repair Activity.Biomed Res Int. 2017;2017:2578017. doi: 10.1155/2017/2578017. Epub 2017 Sep 5. Biomed Res Int. 2017. PMID: 29018809 Free PMC article.
-
Overview of Silk Fibroin Use in Wound Dressings.Trends Biotechnol. 2018 Sep;36(9):907-922. doi: 10.1016/j.tibtech.2018.04.004. Epub 2018 May 12. Trends Biotechnol. 2018. PMID: 29764691 Review.
-
Active wound coverings: bioengineered skin and dermal substitutes.Surg Clin North Am. 2010 Dec;90(6):1237-55. doi: 10.1016/j.suc.2010.08.004. Surg Clin North Am. 2010. PMID: 21074039 Review.
Cited by
-
Extracellular Matrices as Bioactive Materials for In Situ Tissue Regeneration.Pharmaceutics. 2023 Dec 13;15(12):2771. doi: 10.3390/pharmaceutics15122771. Pharmaceutics. 2023. PMID: 38140112 Free PMC article. Review.
-
Skin Inflammation with a Focus on Wound Healing.Adv Wound Care (New Rochelle). 2023 May;12(5):269-287. doi: 10.1089/wound.2021.0126. Epub 2022 May 26. Adv Wound Care (New Rochelle). 2023. PMID: 35287486 Free PMC article. Review.
-
Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction.Stem Cell Res Ther. 2021 Jan 7;12(1):13. doi: 10.1186/s13287-020-02066-y. Stem Cell Res Ther. 2021. PMID: 33413626 Free PMC article.
-
Acellular bovine pericardium as a biological dressing for treatment of cutaneous wounds of the distal limb in donkeys (Equus Asinus).Vet Res Commun. 2023 Jun;47(2):587-597. doi: 10.1007/s11259-022-10014-9. Epub 2022 Nov 3. Vet Res Commun. 2023. PMID: 36323838 Free PMC article.
-
Bioactive Hydrogel Based on Collagen and Hyaluronic Acid Enriched with Freeze-Dried Sheep Placenta for Wound Healing Support.Int J Mol Sci. 2024 Jan 30;25(3):1687. doi: 10.3390/ijms25031687. Int J Mol Sci. 2024. PMID: 38338964 Free PMC article.
References
-
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874. - PubMed
-
- Clark R.A. Ghosh K. Tonnesen M.G. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;127:1018. - PubMed
-
- Choi J.S. Yang H.J. Kim B.S. Kim J.D. Kim J.Y. Yoo B., et al. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

