Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential
- PMID: 22891282
- PMCID: PMC3459682
- DOI: 10.4049/jimmunol.1201053
Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential
Abstract
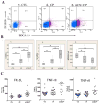
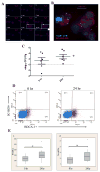
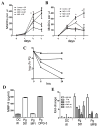
The low-grade oral infection chronic periodontitis (CP) has been implicated in coronary artery disease risk, but the mechanisms are unclear. In this study, a pathophysiological role for blood dendritic cells (DCs) in systemic dissemination of oral mucosal pathogens to atherosclerotic plaques was investigated in humans. The frequency and microbiome of CD19(-)BDCA-1(+)DC-SIGN(+) blood myeloid DCs (mDCs) were analyzed in CP subjects with or without existing acute coronary syndrome and in healthy controls. FACS analysis revealed a significant increase in blood mDCs in the following order: healthy controls < CP < acute coronary syndrome/CP. Analysis of the blood mDC microbiome by 16S rDNA sequencing showed Porphyromonas gingivalis and other species, including (cultivable) Burkholderia cepacia. The mDC carriage rate with P. gingivalis correlated with oral carriage rate and with serologic exposure to P. gingivalis in CP subjects. Intervention (local debridement) to elicit a bacteremia increased the mDC carriage rate and frequency in vivo. In vitro studies established that P. gingivalis enhanced by 28% the differentiation of monocytes into immature mDCs; moreover, mDCs secreted high levels of matrix metalloproteinase-9 and upregulated C1q, heat shock protein 60, heat shock protein 70, CCR2, and CXCL16 transcripts in response to P. gingivalis in a fimbriae-dependent manner. Moreover, the survival of the anaerobe P. gingivalis under aerobic conditions was enhanced when within mDCs. Immunofluorescence analysis of oral mucosa and atherosclerotic plaques demonstrate infiltration with mDCs, colocalized with P. gingivalis. Our results suggest a role for blood mDCs in harboring and disseminating pathogens from oral mucosa to atherosclerosis plaques, which may provide key signals for mDC differentiation and atherogenic conversion.
Conflict of interest statement
The authors have no financial conflicts of interest
Figures


Similar articles
-
Secondary lymphoid organ homing phenotype of human myeloid dendritic cells disrupted by an intracellular oral pathogen.Infect Immun. 2014 Jan;82(1):101-11. doi: 10.1128/IAI.01157-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126519 Free PMC article.
-
Noncanonical dendritic cell differentiation and survival driven by a bacteremic pathogen.J Leukoc Biol. 2013 Aug;94(2):281-9. doi: 10.1189/jlb.0213108. Epub 2013 May 31. J Leukoc Biol. 2013. PMID: 23729500 Free PMC article.
-
Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic Cell Proliferation.Sci Rep. 2018 Nov 9;8(1):16607. doi: 10.1038/s41598-018-35126-8. Sci Rep. 2018. PMID: 30413788 Free PMC article.
-
Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis.Trends Microbiol. 1995 Feb;3(2):45-51. doi: 10.1016/s0966-842x(00)88874-5. Trends Microbiol. 1995. PMID: 7728384 Review.
-
The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay.Microbiology (Reading). 2008 Oct;154(Pt 10):2897-2903. doi: 10.1099/mic.0.2008/021220-0. Microbiology (Reading). 2008. PMID: 18832296 Free PMC article. Review.
Cited by
-
Intratumoral microbiota: a new force in the development and treatment of esophageal cancer.Clin Transl Oncol. 2024 Oct 25. doi: 10.1007/s12094-024-03757-1. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39455494 Review.
-
Characteristics of the oral microbiome in youth exposed to caregiving adversity.Brain Behav Immun Health. 2024 Aug 28;41:100850. doi: 10.1016/j.bbih.2024.100850. eCollection 2024 Nov. Brain Behav Immun Health. 2024. PMID: 39280088 Free PMC article.
-
The oral-gut microbiome axis in health and disease.Nat Rev Microbiol. 2024 Jul 22. doi: 10.1038/s41579-024-01075-5. Online ahead of print. Nat Rev Microbiol. 2024. PMID: 39039286 Review.
-
Oral Microbially-Induced Small Extracellular Vesicles Cross the Blood-Brain Barrier.Int J Mol Sci. 2024 Apr 20;25(8):4509. doi: 10.3390/ijms25084509. Int J Mol Sci. 2024. PMID: 38674094 Free PMC article.
-
Bioinformatics reveals the pathophysiological relationship between diabetic nephropathy and periodontitis in the context of aging.Heliyon. 2024 Jan 18;10(2):e24872. doi: 10.1016/j.heliyon.2024.e24872. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38304805 Free PMC article.
References
-
- Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VL, Badimon J, Goldstein JA, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. - PubMed
-
- Vita JA, Loscalzo J. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation. 2002;106:164–166. - PubMed
-
- Pesonen E, El-Segaier M, Persson K, Puolakkainen M, Sarna S, Ohlin H, Pussinen PJ. Infections as a stimulus for coronary occlusion, obstruction, or acute coronary syndromes. Ther Adv Cardiovasc Dis. 2009;3:447–454. - PubMed
-
- Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J Dent Res. 2006;85:106–121. - PubMed
-
- Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, Couper DJ, Stewart DD, Falkner KL, Graham SP, Grossi S, Gunsolley JC, Madden T, Maupome G, Trevisan M, Van Dyke TE, Genco RJ. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80:190–201. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

