Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation
- PMID: 22886301
- PMCID: PMC3428080
- DOI: 10.1172/JCI61209
Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation
Abstract
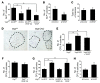
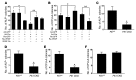
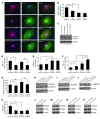
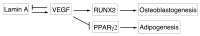
Osteoporotic bones have reduced spongy bone mass, altered bone architecture, and increased marrow fat. Bone marrow stem cells from osteoporotic patients are more likely to differentiate into adipocytes than control cells, suggesting that adipocyte differentiation may play a role in osteoporosis. VEGF is highly expressed in osteoblastic precursor cells and is known to stimulate bone formation. Here we tested the hypothesis that VEGF is also an important regulator of cell fate, determining whether differentiation gives rise to osteoblasts or adipocytes. Mice with conditional VEGF deficiency in osteoblastic precursor cells exhibited an osteoporosis-like phenotype characterized by reduced bone mass and increased bone marrow fat. In addition, reduced VEGF expression in mesenchymal stem cells resulted in reduced osteoblast and increased adipocyte differentiation. Osteoblast differentiation was reduced when VEGF receptor 1 or 2 was knocked down but was unaffected by treatment with recombinant VEGF or neutralizing antibodies against VEGF. Our results suggested that VEGF controls differentiation in mesenchymal stem cells by regulating the transcription factors RUNX2 and PPARγ2 as well as through a reciprocal interaction with nuclear envelope proteins lamin A/C. Importantly, our data support a model whereby VEGF regulates differentiation through an intracrine mechanism that is distinct from the role of secreted VEGF and its receptors.
Figures
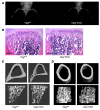
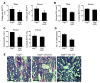
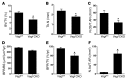
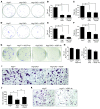
Similar articles
-
Regulation of adipogenesis and osteogenesis in mesenchymal stem cells by vascular endothelial growth factor A.J Intern Med. 2015 Jun;277(6):674-80. doi: 10.1111/joim.12364. J Intern Med. 2015. PMID: 25779338
-
How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells.J Histochem Cytochem. 2014 Feb;62(2):103-8. doi: 10.1369/0022155413516347. Epub 2013 Dec 5. J Histochem Cytochem. 2014. PMID: 24309509 Free PMC article.
-
A novel PPARγ2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation.Cell Death Differ. 2014 Oct;21(10):1642-55. doi: 10.1038/cdd.2014.80. Epub 2014 Jun 20. Cell Death Differ. 2014. PMID: 24948012 Free PMC article.
-
Distinct VEGF functions during bone development and homeostasis.Arch Immunol Ther Exp (Warsz). 2014 Oct;62(5):363-8. doi: 10.1007/s00005-014-0285-y. Epub 2014 Apr 4. Arch Immunol Ther Exp (Warsz). 2014. PMID: 24699630 Review.
-
Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program.Cell Mol Life Sci. 2009 Jan;66(2):236-53. doi: 10.1007/s00018-008-8429-z. Cell Mol Life Sci. 2009. PMID: 18854943 Free PMC article. Review.
Cited by
-
Chronic Intermittent Hypobaric Hypoxia Enhances Bone Fracture Healing.Front Endocrinol (Lausanne). 2021 Feb 16;11:582670. doi: 10.3389/fendo.2020.582670. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33664707 Free PMC article.
-
Use of mesenchymal stem cells for therapy of cardiac disease.Circ Res. 2015 Apr 10;116(8):1413-30. doi: 10.1161/CIRCRESAHA.116.303614. Circ Res. 2015. PMID: 25858066 Free PMC article. Review.
-
From Stem Cells to Bone-Forming Cells.Int J Mol Sci. 2021 Apr 13;22(8):3989. doi: 10.3390/ijms22083989. Int J Mol Sci. 2021. PMID: 33924333 Free PMC article. Review.
-
Response to Comment on: Elias et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes 2012;61:1801-1813.Diabetes. 2013 Jan;62(1):e4. doi: 10.2337/db12-1274. Diabetes. 2013. PMID: 23258920 Free PMC article. No abstract available.
-
Osteogenic and angiogenic characterization of mandible and femur osteoblasts.J Mol Histol. 2019 Apr;50(2):105-117. doi: 10.1007/s10735-019-09810-6. Epub 2019 Jan 11. J Mol Histol. 2019. PMID: 30635760
References
-
- Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79(4):557–565. doi: 10.1002/1097-4644(20001215)79:4<557::AID-JCB40>3.0.CO;2-H. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

