EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors
- PMID: 22879388
- PMCID: PMC3463738
- DOI: 10.1158/1078-0432.CCR-12-1985
EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors
Abstract
Purpose: The Ewing sarcoma family of tumors (ESFT) comprises a group of aggressive, malignant bone, and soft tissue tumors that predominantly affect children and young adults. These tumors frequently share expression of the EWS-FLI-1 translocation, which is central to tumor survival but not present in healthy cells. In this study, we examined EWS-FLI-1 antigens for their capacity to induce immunity against a range of ESFT types.
Design: Computer prediction analysis of peptide binding, HLA-A2.1 stabilization assays, and induction of cytotoxic T-lymphocytes (CTL) in immunized HLA-A2.1 transgenic mice were used to assess the immunogenicity of native and modified peptides derived from the fusion region of EWS-FLI-1 type 1. CTL-killing of multiple ESFT family members in vitro, and control of established xenografts in vivo, was assessed. We also examined whether these peptides could induce human CTLs in vitro.
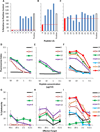
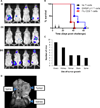
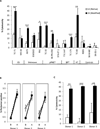
Results: EWS-FLI-1 type 1 peptides were unable to stabilize cell surface HLA-A2.1 and induced weak CTL activity against Ewing sarcoma cells. In contrast, peptides with modified anchor residues induced potent CTL killing of Ewing sarcoma cells presenting endogenous (native) peptides. The adoptive transfer of CTL specific for the modified peptide YLNPSVDSV resulted in enhanced survival of mice with established Ewing sarcoma xenografts. YLNPSVDSV-specific CTL displayed potent killing of multiple ESFT types in vitro: Ewing sarcoma, pPNET, Askin's Tumor, and Biphenotypic sarcoma. Stimulation of human peripheral blood mononuclear cells with YLNPSVDSV peptide resulted in potent CTL-killing.
Conclusions: These data show that YLNPSVDSV peptide is a promising antigen for ESFT immunotherapy and warrants further clinical development.
Conflict of interest statement
The authors disclose no potential conflicts of interest
Figures
Similar articles
-
EWS/FLI-l peptide-pulsed dendritic cells induces the antitumor immunity in a murine Ewing's sarcoma cell model.Int Immunopharmacol. 2014 Aug;21(2):336-41. doi: 10.1016/j.intimp.2014.05.013. Epub 2014 May 24. Int Immunopharmacol. 2014. PMID: 24861249
-
Caveolin-1 (CAV1) is a target of EWS/FLI-1 and a key determinant of the oncogenic phenotype and tumorigenicity of Ewing's sarcoma cells.Cancer Res. 2006 Oct 15;66(20):9937-47. doi: 10.1158/0008-5472.CAN-06-0927. Cancer Res. 2006. PMID: 17047056
-
iPSC-Derived Neoantigen-Specific CTL Therapy for Ewing Sarcoma.Cancer Immunol Res. 2021 Oct;9(10):1175-1186. doi: 10.1158/2326-6066.CIR-21-0193. Epub 2021 Aug 12. Cancer Immunol Res. 2021. PMID: 34385178
-
Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting.Oncotarget. 2016 Apr 5;7(14):17616-30. doi: 10.18632/oncotarget.7124. Oncotarget. 2016. PMID: 26848860 Free PMC article. Review.
-
Ewing sarcoma family of tumors.Adv Anat Pathol. 2005 Jul;12(4):212-20. doi: 10.1097/01.pap.0000175114.55541.52. Adv Anat Pathol. 2005. PMID: 16096383 Review.
Cited by
-
TCR-transgenic T cells and YB-1-based oncolytic virotherapy improve survival in a preclinical Ewing sarcoma xenograft mouse model.Front Immunol. 2024 Jan 22;15:1330868. doi: 10.3389/fimmu.2024.1330868. eCollection 2024. Front Immunol. 2024. PMID: 38318175 Free PMC article.
-
Expression of CCL21 in Ewing sarcoma shows an inverse correlation with metastases and is a candidate target for immunotherapy.Cancer Immunol Immunother. 2016 Aug;65(8):995-1002. doi: 10.1007/s00262-016-1862-1. Epub 2016 Jul 1. Cancer Immunol Immunother. 2016. PMID: 27369431 Free PMC article.
-
Targeting the undruggable: exploiting neomorphic features of fusion oncoproteins in childhood sarcomas for innovative therapies.Cancer Metastasis Rev. 2019 Dec;38(4):625-642. doi: 10.1007/s10555-019-09839-9. Cancer Metastasis Rev. 2019. PMID: 31970591 Free PMC article. Review.
-
Common Ewing sarcoma-associated antigens fail to induce natural T cell responses in both patients and healthy individuals.Cancer Immunol Immunother. 2014 Oct;63(10):1047-60. doi: 10.1007/s00262-014-1574-3. Epub 2014 Jun 28. Cancer Immunol Immunother. 2014. PMID: 24973179 Free PMC article.
-
Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis.Front Oncol. 2019 Jul 3;9:605. doi: 10.3389/fonc.2019.00605. eCollection 2019. Front Oncol. 2019. PMID: 31334125 Free PMC article.
References
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. - PubMed
-
- Lessnick SL, Dei Tos AP, Sorensen PH, Dileo P, Baker LH, Ferrari S, et al. Small round cell sarcomas. Seminars in oncology. 2009;36:338–346. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. The New England journal of medicine. 2003;348:694–701. - PubMed
-
- Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing's sarcoma: standard and experimental treatment options. Current treatment options in oncology. 2009;10:126–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

