Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission
- PMID: 22869886
- PMCID: PMC3434986
- DOI: 10.1200/JCO.2011.41.3807
Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission
Abstract
Purpose: Anti-GD2 monoclonal antibody (MoAb) combined with granulocyte-macrophage colony-stimulating factor (GM-CSF) has shown efficacy against neuroblastoma (NB). Prognostic variables that could influence clinical outcome were explored.
Patients and methods: One hundred sixty-nine children diagnosed with stage 4 NB (1988 to 2008) were enrolled onto consecutive anti-GD2 murine MoAb 3F8 ± GM-CSF ± 13-cis-retinoic acid (CRA) protocols after achieving first remission (complete remission/very good partial remission). Patients enrolled in regimen A (n = 43 high-risk [HR] patients) received 3F8 alone; regimen B (n = 41 HR patients), 3F8 + intravenous GM-CSF + CRA, after stem-cell transplantation (SCT); and regimen C (n = 85), 3F8 + subcutaneous GM-CSF + CRA, 46 of 85 after SCT, whereas 28 of 85 required additional induction therapy and were deemed ultra high risk (UHR). Marrow minimal residual disease (MRD) was measured by quantitative reverse transcription polymerase chain reaction. Survival probability was calculated by the Kaplan-Meier method, and prognostic variables were analyzed by multivariate Cox regression model.
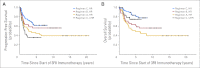
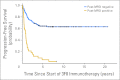
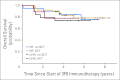
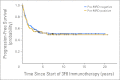
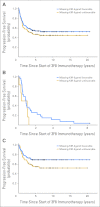
Results: At 5 years from the start of immunotherapy, progression-free survival (PFS) improved from 44% for HR patients receiving regimen A to 56% and 62% for those receiving regimens B and C, respectively. Overall survival (OS) was 49%, 61%, and 81%, respectively. PFS and OS of UHR patients were 36% and 75%, respectively. Relapse was mostly at isolated sites. Independent adverse prognostic factors included UHR (PFS) and post-cycle two MRD (PFS and OS), whereas the prognostic factors for improved outcome were missing killer immunoglobulin-like receptor ligand (PFS and OS), human antimouse antibody response (OS), and regimen C (OS).
Conclusion: Retrospective analysis of consecutive trials from a single center demonstrated that MoAb 3F8 + GM-CSF + CRA is effective against chemotherapy-resistant marrow MRD. Its positive impact on long-term survival can only be confirmed definitively by randomized studies.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
About the benefits of immunotherapy for high-risk neuroblastoma.J Clin Oncol. 2013 Feb 10;31(5):649-50. doi: 10.1200/JCO.2012.47.4080. Epub 2013 Jan 7. J Clin Oncol. 2013. PMID: 23295791 No abstract available.
-
Reply to L. Moreno et al.J Clin Oncol. 2013 Feb 10;31(5):650-1. doi: 10.1200/JCO.2012.47.4593. J Clin Oncol. 2013. PMID: 23520645 No abstract available.
Similar articles
-
Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma.Int J Cancer. 2014 Nov 1;135(9):2199-205. doi: 10.1002/ijc.28851. Epub 2014 Apr 3. Int J Cancer. 2014. PMID: 24644014 Free PMC article. Clinical Trial.
-
Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin.Oncotarget. 2016 Jan 26;7(4):4155-66. doi: 10.18632/oncotarget.6393. Oncotarget. 2016. PMID: 26623730 Free PMC article.
-
Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-GD2 immunotherapy and isotretinoin: a prospective Phase II study.Oncoimmunology. 2015 May 22;4(7):e1016704. doi: 10.1080/2162402X.2015.1016704. eCollection 2015 Jul. Oncoimmunology. 2015. PMID: 26140243 Free PMC article.
-
Targeted immunotherapy for high-risk neuroblastoma--the role of monoclonal antibodies.Ann Pharmacother. 2013 Feb;47(2):210-8. doi: 10.1345/aph.1R353. Epub 2013 Feb 5. Ann Pharmacother. 2013. PMID: 23386066 Review.
-
Evidence for the efficacy of immunotherapy in children with high-risk neuroblastoma.Postepy Hig Med Dosw (Online). 2016 Sep 28;70(0):1001-1004. doi: 10.5604/17322693.1220380. Postepy Hig Med Dosw (Online). 2016. PMID: 27708204 Review.
Cited by
-
Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults.Pediatr Blood Cancer. 2016 Oct;63(10):1780-5. doi: 10.1002/pbc.26097. Epub 2016 Jun 15. Pediatr Blood Cancer. 2016. PMID: 27304202 Free PMC article.
-
Radioimmunotherapy of human tumours.Nat Rev Cancer. 2015 Jun;15(6):347-60. doi: 10.1038/nrc3925. Nat Rev Cancer. 2015. PMID: 25998714 Free PMC article. Review.
-
Mechanisms, Characteristics, and Treatment of Neuropathic Pain and Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients.Int J Mol Sci. 2021 Nov 23;22(23):12648. doi: 10.3390/ijms222312648. Int J Mol Sci. 2021. PMID: 34884452 Free PMC article. Review.
-
Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication.Nat Med. 2022 Feb;28(2):333-344. doi: 10.1038/s41591-021-01625-x. Epub 2022 Jan 13. Nat Med. 2022. PMID: 35027753 Free PMC article.
-
GD2-targeted immunotherapy and radioimmunotherapy.Semin Oncol. 2014 Oct;41(5):589-612. doi: 10.1053/j.seminoncol.2014.07.003. Epub 2014 Jul 21. Semin Oncol. 2014. PMID: 25440605 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

