Effects of selective checkpoint kinase 1 inhibition on cytarabine cytotoxicity in acute myelogenous leukemia cells in vitro
- PMID: 22869869
- PMCID: PMC3463653
- DOI: 10.1158/1078-0432.CCR-12-0961
Effects of selective checkpoint kinase 1 inhibition on cytarabine cytotoxicity in acute myelogenous leukemia cells in vitro
Abstract
Purpose: Previous studies have shown that the replication checkpoint, which involves the kinases ataxia telangiectasia mutated and Rad3 related (ATR) and Chk1, contributes to cytarabine resistance in cell lines. In the present study, we examined whether this checkpoint is activated in clinical acute myelogenous leukemia (AML) during cytarabine infusion in vivo and then assessed the impact of combining cytarabine with the recently described Chk1 inhibitor SCH 900776 in vitro.
Experimental design: AML marrow aspirates harvested before and during cytarabine infusion were examined by immunoblotting. Human AML lines treated with cytarabine in the absence or presence of SCH 900776 were assayed for checkpoint activation by immunoblotting, nucleotide incorporation into DNA, and flow cytometry. Long-term effects in AML lines, clinical AML isolates, and normal myeloid progenitors were assayed using clonogenic assays.
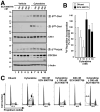
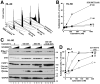
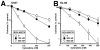
Results: Immunoblotting revealed increased Chk1 phosphorylation, a marker of checkpoint activation, in more than half of Chk1-containing AMLs after 48 hours of cytarabine infusion. In human AML lines, SCH 900776 not only disrupted cytarabine-induced Chk1 activation and S-phase arrest but also markedly increased cytarabine-induced apoptosis. Clonogenic assays demonstrated that SCH 900776 enhanced the antiproliferative effects of cytarabine in AML cell lines and clinical AML samples at concentrations that had negligible impact on normal myeloid progenitors.
Conclusions: These results not only provide evidence for cytarabine-induced S-phase checkpoint activation in AML in the clinical setting, but also show that a selective Chk1 inhibitor can overcome the S-phase checkpoint and enhance the cytotoxicity of cytarabine. Accordingly, further investigation of the cytarabine/SCH 900776 combination in AML appears warranted.
Figures
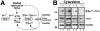
Similar articles
-
Phase I and pharmacologic trial of cytosine arabinoside with the selective checkpoint 1 inhibitor Sch 900776 in refractory acute leukemias.Clin Cancer Res. 2012 Dec 15;18(24):6723-31. doi: 10.1158/1078-0432.CCR-12-2442. Epub 2012 Oct 23. Clin Cancer Res. 2012. PMID: 23092873 Free PMC article. Clinical Trial.
-
CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo.Haematologica. 2014 Apr;99(4):688-96. doi: 10.3324/haematol.2013.093187. Epub 2013 Oct 31. Haematologica. 2014. PMID: 24179152 Free PMC article.
-
Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine.Blood. 2005 Jul 1;106(1):318-27. doi: 10.1182/blood-2004-09-3523. Epub 2005 Mar 22. Blood. 2005. PMID: 15784732 Free PMC article.
-
Novel regulation of checkpoint kinase 1: Is checkpoint kinase 1 a good candidate for anti-cancer therapy?Cancer Sci. 2012 Jul;103(7):1195-200. doi: 10.1111/j.1349-7006.2012.02280.x. Epub 2012 Apr 23. Cancer Sci. 2012. PMID: 22435685 Free PMC article. Review.
-
Checkpoint kinase inhibitors: a patent review (2009 - 2010).Expert Opin Ther Pat. 2011 Aug;21(8):1191-210. doi: 10.1517/13543776.2011.586632. Epub 2011 May 20. Expert Opin Ther Pat. 2011. PMID: 21599421 Review.
Cited by
-
Molecular Threat of Splicing Factor Mutations to Myeloid Malignancies and Potential Therapeutic Modulations.Biomedicines. 2022 Aug 15;10(8):1972. doi: 10.3390/biomedicines10081972. Biomedicines. 2022. PMID: 36009519 Free PMC article. Review.
-
Combined inhibition of Chk1 and Wee1 as a new therapeutic strategy for mantle cell lymphoma.Oncotarget. 2015 Feb 20;6(5):3394-408. doi: 10.18632/oncotarget.2583. Oncotarget. 2015. PMID: 25428911 Free PMC article.
-
Chk1 Inhibitor SCH900776 Effectively Potentiates the Cytotoxic Effects of Platinum-Based Chemotherapeutic Drugs in Human Colon Cancer Cells.Neoplasia. 2017 Oct;19(10):830-841. doi: 10.1016/j.neo.2017.08.002. Epub 2017 Sep 6. Neoplasia. 2017. PMID: 28888100 Free PMC article.
-
Evolution of Molecular Targeted Cancer Therapy: Mechanisms of Drug Resistance and Novel Opportunities Identified by CRISPR-Cas9 Screening.Front Oncol. 2022 Mar 17;12:755053. doi: 10.3389/fonc.2022.755053. eCollection 2022. Front Oncol. 2022. PMID: 35372044 Free PMC article. Review.
-
Targeting the ATR-CHK1 Axis in Cancer Therapy.Cancers (Basel). 2017 Apr 27;9(5):41. doi: 10.3390/cancers9050041. Cancers (Basel). 2017. PMID: 28448462 Free PMC article. Review.
References
-
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy of acute myeloid leukemia. Blood. 2005;106:1154–63. - PubMed
-
- Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. - PubMed
-
- Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31(Pt 2):2349–70. - PubMed
-
- Loegering D, Arlander SAH, Hackbarth J, Vroman B, Lieberman HB, Karnitz LM, et al. Rad9 protects cells from topoisomerase poison-induced cell death. J Biol Chem. 2004;279:18641–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

