Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase
- PMID: 22863787
- PMCID: PMC3491143
- DOI: 10.1038/nm.2885
Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase
Abstract
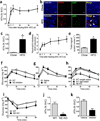
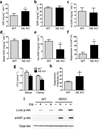
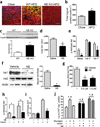
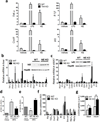
Chronic low-grade adipose tissue and liver inflammation is a major cause of systemic insulin resistance and is a key component of the low degree of insulin sensitivity that exists in obesity and type 2 diabetes. Immune cells, such as macrophages, T cells, B cells, mast cells and eosinophils, have all been implicated as having a role in this process. Neutrophils are typically the first immune cells to respond to inflammation and can exacerbate the chronic inflammatory state by helping to recruit macrophages and by interacting with antigen-presenting cells. Neutrophils secrete several proteases, one of which is neutrophil elastase, which can promote inflammatory responses in several disease models. Here we show that treatment of hepatocytes with neutrophil elastase causes cellular insulin resistance and that deletion of neutrophil elastase in high-fat-diet–induced obese (DIO) mice leads to less tissue inflammation that is associated with lower adipose tissue neutrophil and macrophage content. These changes are accompanied by improved glucose tolerance and increased insulin sensitivity. Taken together, we show that neutrophils can be added to the extensive repertoire of immune cells that participate in inflammation-induced metabolic disease.
Figures
Similar articles
-
The Effects of Poncirus fructus on Insulin Resistance and the Macrophage-Mediated Inflammatory Response in High Fat Diet-Induced Obese Mice.Int J Mol Sci. 2019 Jun 12;20(12):2858. doi: 10.3390/ijms20122858. Int J Mol Sci. 2019. PMID: 31212747 Free PMC article.
-
From neutrophils to macrophages: differences in regional adipose tissue depots.Obes Rev. 2016 Jan;17(1):1-17. doi: 10.1111/obr.12335. Epub 2015 Dec 14. Obes Rev. 2016. PMID: 26667065 Review.
-
Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation.J Immunol. 2014 Oct 15;193(8):4002-9. doi: 10.4049/jimmunol.1401149. Epub 2014 Sep 12. J Immunol. 2014. PMID: 25217161
-
Macrophage migration inhibitory factor deficiency ameliorates high-fat diet induced insulin resistance in mice with reduced adipose inflammation and hepatic steatosis.PLoS One. 2014 Nov 20;9(11):e113369. doi: 10.1371/journal.pone.0113369. eCollection 2014. PLoS One. 2014. PMID: 25412423 Free PMC article.
-
Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue.Proc Nutr Soc. 2011 Nov;70(4):408-17. doi: 10.1017/S0029665111000565. Epub 2011 Aug 12. Proc Nutr Soc. 2011. PMID: 21835098 Review.
Cited by
-
Transcriptional regulation of chemokine genes: a link to pancreatic islet inflammation?Biomolecules. 2015 May 26;5(2):1020-34. doi: 10.3390/biom5021020. Biomolecules. 2015. PMID: 26018641 Free PMC article. Review.
-
The Immune Landscape in Nonalcoholic Steatohepatitis.Immune Netw. 2016 Jun;16(3):147-58. doi: 10.4110/in.2016.16.3.147. Epub 2016 Jun 17. Immune Netw. 2016. PMID: 27340383 Free PMC article. Review.
-
Role of the chemokine system in liver fibrosis: a narrative review.Dig Med Res. 2022 Jun;5:30. doi: 10.21037/dmr-21-87. Epub 2022 Jun 30. Dig Med Res. 2022. PMID: 36339901 Free PMC article.
-
Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model.Nutrients. 2016 Jul 2;8(7):410. doi: 10.3390/nu8070410. Nutrients. 2016. PMID: 27384583 Free PMC article.
-
The Roles of Neutrophils in the Pathogenesis of Liver Diseases.Front Immunol. 2021 Mar 8;12:625472. doi: 10.3389/fimmu.2021.625472. eCollection 2021. Front Immunol. 2021. PMID: 33763069 Free PMC article. Review.
References
-
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. - PubMed
-
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. - PubMed
-
- Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK033651/DK/NIDDK NIH HHS/United States
- R24 DK090962/DK/NIDDK NIH HHS/United States
- P01 DK074868/DK/NIDDK NIH HHS/United States
- T32 DK 007494/DK/NIDDK NIH HHS/United States
- R01 DK033651/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- T32 DK007494/DK/NIDDK NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- DK074868/DK/NIDDK NIH HHS/United States
- P50 HD012303/HD/NICHD NIH HHS/United States
- DK 090962/DK/NIDDK NIH HHS/United States
- U54 HD 012303-25/HD/NICHD NIH HHS/United States
- U54 HD012303/HD/NICHD NIH HHS/United States
- R37 DK033651/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

