Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts
- PMID: 22859370
- PMCID: PMC3475245
- DOI: 10.1096/fj.12-213496
Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts
Abstract
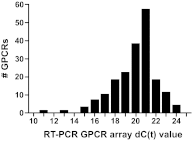
G-protein-coupled receptors (GPCRs) have many roles in cell regulation and are commonly used as drug targets, but the repertoire of GPCRs expressed by individual cell types has not been defined. Here we use an unbiased approach, GPCR RT-PCR array, to define the expression of nonchemosensory GPCRs by cardiac fibroblasts (CFs) isolated from Rattus norvegicus. CFs were selected because of their importance for cardiac structure and function and their contribution to cardiac fibrosis, which occurs with advanced age, after acute injury (e.g., myocardial infarction), and in disease states (e.g., diabetes mellitus, hypertension). We discovered that adult rat CFs express 190 GPCRs and that activation of protease-activated receptor 1 (PAR1), the most highly expressed receptor, raises the expression of profibrotic markers in rat CFs, resulting in a 60% increase in collagen synthesis and conversion to a profibrogenic myofibroblast phenotype. We use siRNA knockdown of PAR1 (90% decrease in mRNA) to show that the profibrotic effects of thrombin are PAR1-dependent. These findings, which define the expression of GPCRs in CFs, provide a proof of principle of an approach to discover previously unappreciated, functionally relevant GPCRs and reveal a potential role for thrombin and PAR1 in wound repair and pathophysiology of the adult heart.
Figures
Similar articles
-
SCH 79797, a selective PAR1 antagonist, limits myocardial ischemia/reperfusion injury in rat hearts.Basic Res Cardiol. 2007 Jul;102(4):350-8. doi: 10.1007/s00395-007-0653-4. Epub 2007 Apr 30. Basic Res Cardiol. 2007. PMID: 17468933 Free PMC article.
-
N-linked glycosylation of protease-activated receptor-1 at extracellular loop 2 regulates G-protein signaling bias.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3600-8. doi: 10.1073/pnas.1508838112. Epub 2015 Jun 22. Proc Natl Acad Sci U S A. 2015. PMID: 26100877 Free PMC article.
-
Coagulation Factor Xa Induces Proinflammatory Responses in Cardiac Fibroblasts via Activation of Protease-Activated Receptor-1.Cells. 2021 Oct 30;10(11):2958. doi: 10.3390/cells10112958. Cells. 2021. PMID: 34831181 Free PMC article.
-
GPCR expression in the heart; "new" receptors in myocytes and fibroblasts.Trends Cardiovasc Med. 2004 Apr;14(3):94-9. doi: 10.1016/j.tcm.2003.12.007. Trends Cardiovasc Med. 2004. PMID: 15121156 Review.
-
Targeting GPCRs to treat cardiac fibrosis.Front Cardiovasc Med. 2022 Oct 6;9:1011176. doi: 10.3389/fcvm.2022.1011176. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277752 Free PMC article. Review.
Cited by
-
Are anticoagulants still indicated in pulmonary arterial hypertension?Pulm Circ. 2018 Oct-Dec;8(4):2045894018807681. doi: 10.1177/2045894018807681. Epub 2018 Oct 4. Pulm Circ. 2018. PMID: 30284508 Free PMC article.
-
Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis.Am J Physiol Cell Physiol. 2014 May 1;306(9):C779-88. doi: 10.1152/ajpcell.00381.2013. Epub 2013 Dec 18. Am J Physiol Cell Physiol. 2014. PMID: 24352335 Free PMC article. Review.
-
Cardiac Expression of Factor X Mediates Cardiac Hypertrophy and Fibrosis in Pressure Overload.JACC Basic Transl Sci. 2020 Jan 27;5(1):69-83. doi: 10.1016/j.jacbts.2019.10.006. eCollection 2020 Jan. JACC Basic Transl Sci. 2020. Retraction in: JACC Basic Transl Sci. 2022 Sep 26;7(9):970-971. doi: 10.1016/j.jacbts.2022.08.001 PMID: 32043021 Free PMC article. Retracted.
-
Phos-tag SDS-PAGE resolves agonist- and isoform-specific activation patterns for PKD2 and PKD3 in cardiomyocytes and cardiac fibroblasts.J Mol Cell Cardiol. 2016 Oct;99:14-22. doi: 10.1016/j.yjmcc.2016.08.005. Epub 2016 Aug 8. J Mol Cell Cardiol. 2016. PMID: 27515283 Free PMC article.
-
GPCRomics: An Approach to Discover GPCR Drug Targets.Trends Pharmacol Sci. 2019 Jun;40(6):378-387. doi: 10.1016/j.tips.2019.04.001. Epub 2019 May 8. Trends Pharmacol Sci. 2019. PMID: 31078319 Free PMC article. Review.
References
-
- Gabbiani G. (2003) The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 - PubMed
-
- Hunyady L., Catt K. J. (2006) Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 20, 953–970 - PubMed
-
- Ruster C., Wolf G. (2011) Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J. Am. Soc. Nephrol. 22, 1189–1199 - PubMed
-
- Vignon-Zellweger N., Heiden S., Miyauchi T., Emoto N. (2012) Endothelin and endothelin receptors in the renal and cardiovascular systems. [E-pub ahead of print] Life Sci. doi: 10.1016/j.lfs.2012.03.026 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

