Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations
- PMID: 22855672
- PMCID: PMC3405297
- DOI: 10.3389/fnana.2012.00029
Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations
Abstract
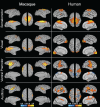
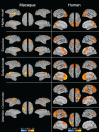
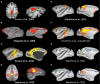
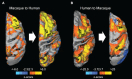
Resting-state investigations based on the evaluation of intrinsic low-frequency fluctuations of the BOLD fMRI signal have been extensively utilized to map the structure and dynamics of large-scale functional network organization in humans. In addition to increasing our knowledge of normal brain connectivity, disruptions of the spontaneous hemodynamic fluctuations have been suggested as possible diagnostic indicators of neurological and psychiatric disease states. Though the non-invasive technique has been received with much acclamation, open questions remain regarding the origin, organization, phylogenesis, as well as the basis of disease-related alterations underlying the signal patterns. Experimental work utilizing animal models, including the use of neurophysiological recordings and pharmacological manipulations, therefore, represents a critical component in the understanding and successful application of resting-state analysis, as it affords a range of experimental manipulations not possible in human subjects. In this article, we review recent rodent and non-human primate studies and based on the examination of the homologous brain architecture propose the latter to be the best-suited model for exploring these unresolved resting-state concerns. Ongoing work examining the correspondence of functional and structural connectivity, state-dependency and the neuronal correlates of the hemodynamic oscillations are discussed. We then consider the potential experiments that will allow insight into different brain states and disease-related network disruptions that can extend the clinical applications of resting-state fMRI (RS-fMRI).
Keywords: animal model; functional MRI (fMRI); functional connectivity; macaque; non-human primate; resting-state; spontaneous activity.
Figures

Similar articles
-
Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest.Hum Brain Mapp. 2008 Jul;29(7):751-61. doi: 10.1002/hbm.20580. Hum Brain Mapp. 2008. PMID: 18465799 Free PMC article.
-
Biophysical and neural basis of resting state functional connectivity: Evidence from non-human primates.Magn Reson Imaging. 2017 Jun;39:71-81. doi: 10.1016/j.mri.2017.01.020. Epub 2017 Feb 2. Magn Reson Imaging. 2017. PMID: 28161319 Free PMC article. Review.
-
Functional Connectivity Mapping in the Animal Model: Principles and Applications of Resting-State fMRI.Front Neurol. 2017 May 10;8:200. doi: 10.3389/fneur.2017.00200. eCollection 2017. Front Neurol. 2017. PMID: 28539914 Free PMC article. Review.
-
Broadband Electrophysiological Dynamics Contribute to Global Resting-State fMRI Signal.J Neurosci. 2016 Jun 1;36(22):6030-40. doi: 10.1523/JNEUROSCI.0187-16.2016. J Neurosci. 2016. PMID: 27251624 Free PMC article.
-
Evaluation of Resting Spatio-Temporal Dynamics of a Neural Mass Model Using Resting fMRI Connectivity and EEG Microstates.Front Comput Neurosci. 2020 Jan 17;13:91. doi: 10.3389/fncom.2019.00091. eCollection 2019. Front Comput Neurosci. 2020. PMID: 32009922 Free PMC article.
Cited by
-
A Putative Multiple-Demand System in the Macaque Brain.J Neurosci. 2016 Aug 17;36(33):8574-85. doi: 10.1523/JNEUROSCI.0810-16.2016. J Neurosci. 2016. PMID: 27535906 Free PMC article.
-
Cross-species functional alignment reveals evolutionary hierarchy within the connectome.Neuroimage. 2020 Dec;223:117346. doi: 10.1016/j.neuroimage.2020.117346. Epub 2020 Sep 9. Neuroimage. 2020. PMID: 32916286 Free PMC article.
-
Hippocampal connectivity patterns echo macroscale cortical evolution in the primate brain.Nat Commun. 2024 Jul 16;15(1):5963. doi: 10.1038/s41467-024-49823-8. Nat Commun. 2024. PMID: 39013855 Free PMC article.
-
Correlated inter-regional variations in low frequency local field potentials and resting state BOLD signals within S1 cortex of monkeys.Hum Brain Mapp. 2016 Aug;37(8):2755-66. doi: 10.1002/hbm.23207. Epub 2016 Apr 19. Hum Brain Mapp. 2016. PMID: 27091582 Free PMC article.
-
Functional connectivity across the human subcortical auditory system using an autoregressive matrix-Gaussian copula graphical model approach with partial correlations.Imaging Neurosci (Camb). 2024;2:10.1162/imag_a_00258. doi: 10.1162/imag_a_00258. Epub 2024 Aug 12. Imaging Neurosci (Camb). 2024. PMID: 39421593 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

