Autophagy in proximal tubules protects against acute kidney injury
- PMID: 22854643
- PMCID: PMC3491167
- DOI: 10.1038/ki.2012.261
Autophagy in proximal tubules protects against acute kidney injury
Abstract
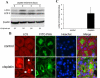
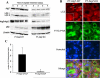
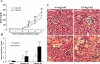
Autophagy is induced in renal tubular cells during acute kidney injury; however, whether this is protective or injurious remains controversial. We address this question by pharmacologic and genetic blockade of autophagy using mouse models of cisplatin- and ischemia-reperfusion-induced acute kidney injury. Chloroquine, a pharmacological inhibitor of autophagy, blocked autophagic flux and enhanced acute kidney injury in both models. Rapamycin, however, activated autophagy and protected against cisplatin-induced acute kidney injury. We also established a renal proximal tubule-specific autophagy-related gene 7-knockout mouse model shown to be defective in both basal and cisplatin-induced autophagy in kidneys. Compared with wild-type littermates, these knockout mice were markedly more sensitive to cisplatin-induced acute kidney injury as indicated by renal functional loss, tissue damage, and apoptosis. Mechanistically, these knockout mice had heightened activation of p53 and c-Jun N terminal kinase, the signaling pathways contributing to cisplatin acute kidney injury. Proximal tubular cells isolated from the knockout mice were more sensitive to cisplatin-induced apoptosis than cells from wild-type mice. In addition, the knockout mice were more sensitive to renal ischemia-reperfusion injury than their wild-type littermates. Thus, our results establish a renoprotective role of tubular cell autophagy in acute kidney injury where it may interfere with cell killing mechanisms.
Figures





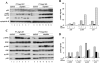
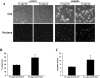
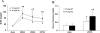


Comment in
-
Autophagy protects proximal tubular cells from injury and apoptosis.Kidney Int. 2012 Dec;82(12):1250-3. doi: 10.1038/ki.2012.337. Kidney Int. 2012. PMID: 23203020 Free PMC article.
Similar articles
-
Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells.Cell Death Dis. 2018 Feb 23;9(3):322. doi: 10.1038/s41419-018-0374-7. Cell Death Dis. 2018. PMID: 29476062 Free PMC article.
-
PINK1/Parkin-mediated mitophagy is activated in cisplatin nephrotoxicity to protect against kidney injury.Cell Death Dis. 2018 Nov 1;9(11):1113. doi: 10.1038/s41419-018-1152-2. Cell Death Dis. 2018. PMID: 30385753 Free PMC article.
-
Interferon-γ is protective in cisplatin-induced renal injury by enhancing autophagic flux.Kidney Int. 2012 Nov;82(10):1093-104. doi: 10.1038/ki.2012.240. Epub 2012 Jul 11. Kidney Int. 2012. PMID: 22785177
-
Autophagy in acute kidney injury.Semin Nephrol. 2014 Jan;34(1):17-26. doi: 10.1016/j.semnephrol.2013.11.004. Epub 2013 Nov 21. Semin Nephrol. 2014. PMID: 24485026 Free PMC article. Review.
-
Autophagy in acute kidney injury and repair.Nephron Clin Pract. 2014;127(1-4):56-60. doi: 10.1159/000363677. Epub 2014 Sep 24. Nephron Clin Pract. 2014. PMID: 25343822 Free PMC article. Review.
Cited by
-
Mouse model of ischemic acute kidney injury: technical notes and tricks.Am J Physiol Renal Physiol. 2012 Dec 1;303(11):F1487-94. doi: 10.1152/ajprenal.00352.2012. Epub 2012 Sep 19. Am J Physiol Renal Physiol. 2012. PMID: 22993069 Free PMC article. Review.
-
ALDH2 modulates autophagy flux to regulate acetaldehyde-mediated toxicity thresholds.Am J Cancer Res. 2016 Mar 15;6(4):781-96. eCollection 2016. Am J Cancer Res. 2016. PMID: 27186430 Free PMC article.
-
Heat shock factor 1 induces crystallin-αB to protect against cisplatin nephrotoxicity.Am J Physiol Renal Physiol. 2016 Jul 1;311(1):F94-F102. doi: 10.1152/ajprenal.00201.2016. Epub 2016 May 18. Am J Physiol Renal Physiol. 2016. PMID: 27194715 Free PMC article.
-
Energy metabolic reprogramming regulates programmed cell death of renal tubular epithelial cells and might serve as a new therapeutic target for acute kidney injury.Front Cell Dev Biol. 2023 Nov 20;11:1276217. doi: 10.3389/fcell.2023.1276217. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38054182 Free PMC article. Review.
-
Kaempferide ameliorates cisplatin-induced nephrotoxicity via inhibiting oxidative stress and inducing autophagy.Acta Pharmacol Sin. 2023 Jul;44(7):1442-1454. doi: 10.1038/s41401-023-01051-4. Epub 2023 Jan 19. Acta Pharmacol Sin. 2023. PMID: 36658427 Free PMC article.
References
-
- Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. - PubMed
-
- Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. - PubMed
-
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

