c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart
- PMID: 22847442
- PMCID: PMC3421216
- DOI: 10.1073/pnas.1208114109
c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart
Abstract
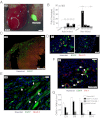
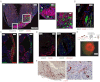
We examined the myogenic response to infarction in neonatal and adult mice to determine the role of c-kit(+) cardiovascular precursor cells (CPC) that are known to be present in early heart development. Infarction of postnatal day 1-3 c-kit(BAC)-EGFP mouse hearts induced the localized expansion of (c-kit)EGFP(+) cells within the infarct, expression of the c-kit and Nkx2.5 mRNA, myogenesis, and partial regeneration of the infarction, with (c-kit)EGFP(+) cells adopting myogenic and vascular fates. Conversely, infarction of adult mice resulted in a modest induction of (c-kit)EGFP(+) cells within the infarct, which did not express Nkx2.5 or undergo myogenic differentiation, but adopted a vascular fate within the infarction, indicating a lack of authentic CPC. Explantation of infarcted neonatal and adult heart tissue to scid mice, and adoptive transfer of labeled bone marrow, confirmed the cardiac source of myogenic (neonate) and angiogenic (neonate and adult) cells. FACS-purified (c-kit)EGFP(+)/(αMHC)mCherry(-) (noncardiac) cells from microdissected infarcts within 6 h of infarction underwent cardiac differentiation, forming spontaneously beating myocytes in vitro; cre/LoxP fate mapping identified a noncardiac population of (c-kit)EGFP(+) myocytes within infarctions, indicating that the induction of undifferentiated precursors contributes to localized myogenesis. Thus, adult postinfarct myogenic failure is likely not due to a context-dependent restriction of precursor differentiation, and c-kit induction following injury of the adult heart does not define precursor status.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Comment on "Do neonatal mouse hearts regenerate following heart apex resection"?Stem Cell Reports. 2014 Jul 8;3(1):2. doi: 10.1016/j.stemcr.2014.06.010. eCollection 2014 Jul 8. Stem Cell Reports. 2014. PMID: 25068115 Free PMC article. No abstract available.
Similar articles
-
c-kit expression identifies cardiovascular precursors in the neonatal heart.Proc Natl Acad Sci U S A. 2009 Feb 10;106(6):1808-13. doi: 10.1073/pnas.0808920106. Epub 2009 Feb 4. Proc Natl Acad Sci U S A. 2009. PMID: 19193854 Free PMC article.
-
Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts.Circulation. 2010 May 11;121(18):1992-2000. doi: 10.1161/CIRCULATIONAHA.109.909093. Epub 2010 Apr 26. Circulation. 2010. PMID: 20421520 Free PMC article.
-
Evolution of the c-kit-positive cell response to pathological challenge in the myocardium.Stem Cells. 2008 May;26(5):1315-24. doi: 10.1634/stemcells.2007-0751. Epub 2008 Feb 28. Stem Cells. 2008. PMID: 18308948 Free PMC article.
-
Concise review: The role of C-kit expressing cells in heart repair at the neonatal and adult stage.Stem Cells. 2014 Jul;32(7):1701-12. doi: 10.1002/stem.1696. Stem Cells. 2014. PMID: 24585704 Review.
-
"String theory" of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results.Circ Res. 2015 Mar 27;116(7):1216-30. doi: 10.1161/CIRCRESAHA.116.305557. Circ Res. 2015. PMID: 25814683 Free PMC article. Review.
Cited by
-
Generation of cardiac progenitor cells through epicardial to mesenchymal transition.J Mol Med (Berl). 2015 Jul;93(7):735-48. doi: 10.1007/s00109-015-1290-2. Epub 2015 May 7. J Mol Med (Berl). 2015. PMID: 25943780 Review.
-
Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure.Eur Heart J. 2016 Jun 14;37(23):1789-98. doi: 10.1093/eurheartj/ehw113. Epub 2016 Apr 7. Eur Heart J. 2016. PMID: 27055812 Free PMC article. Review.
-
Robust small molecule-aided cardiac reprogramming systems selective to cardiac fibroblasts.iScience. 2023 Nov 14;26(12):108466. doi: 10.1016/j.isci.2023.108466. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077137 Free PMC article.
-
Surgical models for cardiac regeneration in neonatal mice.Nat Protoc. 2014 Feb;9(2):305-11. doi: 10.1038/nprot.2014.021. Epub 2014 Jan 16. Nat Protoc. 2014. PMID: 24434799 Free PMC article.
-
CENP-A is essential for cardiac progenitor cell proliferation.Cell Cycle. 2014;13(5):739-48. doi: 10.4161/cc.27549. Epub 2013 Dec 20. Cell Cycle. 2014. PMID: 24362315 Free PMC article.
References
-
- Sussman MA, Anversa P. Myocardial aging and senescence: Where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. - PubMed
-
- Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. - PubMed
-
- Rubart M, Field LJ. Cardiac regeneration: Repopulating the heart. Annu Rev Physiol. 2006;68:29–49. - PubMed
-
- Murry CE, Reinecke H, Pabon LM. Regeneration gaps: Observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

