Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion
- PMID: 22847424
- PMCID: PMC3427065
- DOI: 10.1073/pnas.1211304109
Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion
Abstract
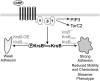
Chemotaxis depends on a network of parallel pathways that coordinate cytoskeletal events to bias cell movement along a chemoattractant gradient. Using a forward genetic screen in Dictyostelium discoideum, we identified the Ste20 kinase KrsB, a homolog of tumor suppressors Hippo and MST1/2, as a negative regulator of cell spreading and substrate attachment. The excessive adhesion of krsB(-) cells reduced directional movement and prolonged the streaming phase of multicellular aggregation. These phenotypes depended on an intact kinase domain and phosphorylation of a conserved threonine (T176) within the activation loop. Chemoattractants triggered a rapid, transient autophosphorylation of T176 in a heterotrimeric G protein-dependent and PI3K- and TorC2-independent manner. The active phosphorylated form of KrsB acts to decrease adhesion to the substrate. Taken together these studies suggest that cycling between active and inactive forms of KrsB may provide the dynamic regulation of cell adhesion needed for proper cell migration and chemotaxis. KrsB interacts genetically with another D. discoideum Hippo/MST homolog, KrsA, but the two genes are not functionally redundant. These studies show that Hippo/MST proteins, like the tumor suppressor PTEN and oncogenes Ras and PI3K, play a key role in cell morphological events in addition to their role in regulating cell growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
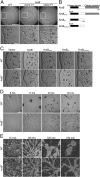
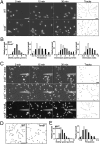
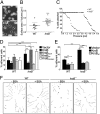

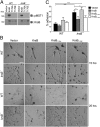
Similar articles
-
Hippo on the move: tumor suppressor regulates adhesion and migration.Cell Cycle. 2013 Feb 15;12(4):535-6. doi: 10.4161/cc.23668. Epub 2013 Jan 31. Cell Cycle. 2013. PMID: 23370390 Free PMC article. No abstract available.
-
The MST4-MOB4 complex disrupts the MST1-MOB1 complex in the Hippo-YAP pathway and plays a pro-oncogenic role in pancreatic cancer.J Biol Chem. 2018 Sep 14;293(37):14455-14469. doi: 10.1074/jbc.RA118.003279. Epub 2018 Aug 2. J Biol Chem. 2018. PMID: 30072378 Free PMC article.
-
PIP3-independent activation of TorC2 and PKB at the cell's leading edge mediates chemotaxis.Curr Biol. 2008 Jul 22;18(14):1034-43. doi: 10.1016/j.cub.2008.06.068. Curr Biol. 2008. PMID: 18635356 Free PMC article.
-
The roles and mechanisms of MST1/2 in the innate immune response.Yi Chuan. 2017 Jul 20;39(7):642-649. doi: 10.16288/j.yczz.17-066. Yi Chuan. 2017. PMID: 28757478 Review.
-
The functions of the Hippo signaling pathway in immune cells.Yi Chuan. 2017 Jul 20;39(7):650-658. doi: 10.16288/j.yczz.17-083. Yi Chuan. 2017. PMID: 28757479 Review.
Cited by
-
The centrosomal component CEP161 of Dictyostelium discoideum interacts with the Hippo signaling pathway.Cell Cycle. 2015;14(7):1024-35. doi: 10.1080/15384101.2015.1007015. Cell Cycle. 2015. PMID: 25607232 Free PMC article.
-
How to understand and outwit adaptation.Dev Cell. 2014 Mar 31;28(6):607-616. doi: 10.1016/j.devcel.2014.03.009. Dev Cell. 2014. PMID: 24697896 Free PMC article.
-
Regulation of a LATS-homolog by Ras GTPases is important for the control of cell division.BMC Cell Biol. 2014 Jul 1;15:25. doi: 10.1186/1471-2121-15-25. BMC Cell Biol. 2014. PMID: 24986648 Free PMC article.
-
Increasing the Content of High-Content Screening: An Overview.J Biomol Screen. 2014 Jun;19(5):640-50. doi: 10.1177/1087057114528537. Epub 2014 Apr 7. J Biomol Screen. 2014. PMID: 24710339 Free PMC article. Review.
-
Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):E7500-E7509. doi: 10.1073/pnas.1608767113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821730 Free PMC article.
References
-
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. - PubMed
-
- Iijima M, Devreotes PN. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. - PubMed
-
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

