Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5
- PMID: 22843785
- PMCID: PMC3657842
- DOI: 10.1161/CIRCRESAHA.111.263525
Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5
Abstract
Rationale: Kv1.5 (KCNA5) is expressed in the heart, where it underlies the I(Kur) current that controls atrial repolarization, and in the pulmonary vasculature, where it regulates vessel contractility in response to changes in oxygen tension. Atrial fibrillation and hypoxic pulmonary hypertension are characterized by downregulation of Kv1.5 protein expression, as well as with oxidative stress. Formation of sulfenic acid on cysteine residues of proteins is an important, dynamic mechanism for protein regulation under oxidative stress. Kv1.5 is widely reported to be redox-sensitive, and the channel possesses 6 potentially redox-sensitive intracellular cysteines. We therefore hypothesized that sulfenic acid modification of the channel itself may regulate Kv1.5 in response to oxidative stress.
Objective: To investigate how oxidative stress, via redox-sensitive modification of the channel with sulfenic acid, regulates trafficking and expression of Kv1.5.
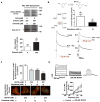
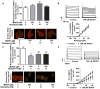
Methods and results: Labeling studies with the sulfenic acid-specific probe DAz and horseradish peroxidase-streptavidin Western blotting demonstrated a global increase in sulfenic acid-modified proteins in human patients with atrial fibrillation, as well as sulfenic acid modification to Kv1.5 in the heart. Further studies showed that Kv1.5 is modified with sulfenic acid on a single COOH-terminal cysteine (C581), and the level of sulfenic acid increases in response to oxidant exposure. Using live-cell immunofluorescence and whole-cell voltage-clamping, we found that modification of this cysteine is necessary and sufficient to reduce channel surface expression, promote its internalization, and block channel recycling back to the cell surface. Moreover, Western blotting demonstrated that sulfenic acid modification is a trigger for channel degradation under prolonged oxidative stress.
Conclusions: Sulfenic acid modification to proteins, which is elevated in diseased human heart, regulates Kv1.5 channel surface expression and stability under oxidative stress and diverts channel from a recycling pathway to degradation. This provides a molecular mechanism linking oxidative stress and downregulation of channel expression observed in cardiovascular diseases.
Figures


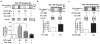
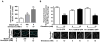


Similar articles
-
Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation.Hum Mol Genet. 2006 Jul 15;15(14):2185-91. doi: 10.1093/hmg/ddl143. Epub 2006 Jun 13. Hum Mol Genet. 2006. PMID: 16772329
-
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.Circ Res. 2014 Mar 14;114(6):982-92. doi: 10.1161/CIRCRESAHA.114.302711. Epub 2014 Feb 7. Circ Res. 2014. PMID: 24508725 Free PMC article.
-
Microtubule polymerization state and clathrin-dependent internalization regulate dynamics of cardiac potassium channel: Microtubule and clathrin control of KV1.5 channel.J Mol Cell Cardiol. 2020 Jul;144:127-139. doi: 10.1016/j.yjmcc.2020.05.004. Epub 2020 May 20. J Mol Cell Cardiol. 2020. PMID: 32445844
-
Challenges Faced with Small Molecular Modulators of Potassium Current Channel Isoform Kv1.5.Biomolecules. 2019 Dec 19;10(1):10. doi: 10.3390/biom10010010. Biomolecules. 2019. PMID: 31861703 Free PMC article. Review.
-
Protein sulfenic acid formation: from cellular damage to redox regulation.Free Radic Biol Med. 2011 Jul 15;51(2):314-26. doi: 10.1016/j.freeradbiomed.2011.04.031. Epub 2011 Apr 23. Free Radic Biol Med. 2011. PMID: 21605662 Review.
Cited by
-
Inhibition of aldose-reductase-2 by a benzofuroxane derivative bf-5m increases the expression of kcne1, kcnq1 in high glucose cultured H9c2 cardiac cells and sudden cardiac death.Oncotarget. 2017 Dec 14;9(25):17257-17269. doi: 10.18632/oncotarget.23270. eCollection 2018 Apr 3. Oncotarget. 2017. PMID: 29707106 Free PMC article.
-
Reactivity, Selectivity, and Stability in Sulfenic Acid Detection: A Comparative Study of Nucleophilic and Electrophilic Probes.Bioconjug Chem. 2016 May 18;27(5):1411-8. doi: 10.1021/acs.bioconjchem.6b00181. Epub 2016 May 9. Bioconjug Chem. 2016. PMID: 27123991 Free PMC article.
-
Protein S-sulfenylation is a fleeting molecular switch that regulates non-enzymatic oxidative folding.Nat Commun. 2016 Aug 22;7:12490. doi: 10.1038/ncomms12490. Nat Commun. 2016. PMID: 27546612 Free PMC article.
-
Biological chemistry and functionality of protein sulfenic acids and related thiol modifications.Free Radic Res. 2016;50(2):172-94. doi: 10.3109/10715762.2015.1090571. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26340608 Free PMC article. Review.
-
Profiling the Reactivity of Cyclic C-Nucleophiles towards Electrophilic Sulfur in Cysteine Sulfenic Acid.Chem Sci. 2016 Jan 1;7(1):400-415. doi: 10.1039/C5SC02569A. Epub 2015 Oct 7. Chem Sci. 2016. PMID: 26819701 Free PMC article.
References
-
- Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73:276–85. - PubMed
-
- Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52:105–20. - PubMed
-
- Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–81. - PubMed
-
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. - PubMed
-
- Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

