The NLRP12 inflammasome recognizes Yersinia pestis
- PMID: 22840842
- PMCID: PMC3753114
- DOI: 10.1016/j.immuni.2012.07.006
The NLRP12 inflammasome recognizes Yersinia pestis
Erratum in
- Immunity. 2012 Sep 21;37(3):588
Abstract
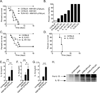
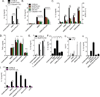
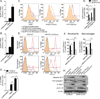
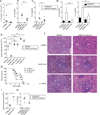
Yersinia pestis, the causative agent of plague, is able to suppress production of inflammatory cytokines IL-18 and IL-1β, which are generated through caspase-1-activating nucleotide-binding domain and leucine-rich repeat (NLR)-containing inflammasomes. Here, we sought to elucidate the role of NLRs and IL-18 during plague. Lack of IL-18 signaling led to increased susceptibility to Y. pestis, producing tetra-acylated lipid A, and an attenuated strain producing a Y. pseudotuberculosis-like hexa-acylated lipid A. We found that the NLRP12 inflammasome was an important regulator controlling IL-18 and IL-1β production after Y. pestis infection, and NLRP12-deficient mice were more susceptible to bacterial challenge. NLRP12 also directed interferon-γ production via induction of IL-18, but had minimal effect on signaling to the transcription factor NF-κB. These studies reveal a role for NLRP12 in host resistance against pathogens. Minimizing NLRP12 inflammasome activation may have been a central factor in evolution of the high virulence of Y. pestis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
The Yersinia pestis Effector YopM Inhibits Pyrin Inflammasome Activation.PLoS Pathog. 2016 Dec 2;12(12):e1006035. doi: 10.1371/journal.ppat.1006035. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27911947 Free PMC article.
-
Manipulation of Interleukin-1β and Interleukin-18 Production by Yersinia pestis Effectors YopJ and YopM and Redundant Impact on Virulence.J Biol Chem. 2016 May 6;291(19):9894-905. doi: 10.1074/jbc.M115.697698. Epub 2016 Feb 16. J Biol Chem. 2016. PMID: 26884330 Free PMC article.
-
A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages.PLoS Pathog. 2011 Apr;7(4):e1002026. doi: 10.1371/journal.ppat.1002026. Epub 2011 Apr 21. PLoS Pathog. 2011. PMID: 21533069 Free PMC article.
-
NLRP12 in innate immunity and inflammation.Mol Aspects Med. 2020 Dec;76:100887. doi: 10.1016/j.mam.2020.100887. Epub 2020 Aug 22. Mol Aspects Med. 2020. PMID: 32838963 Free PMC article. Review.
-
Inflammasomes and host defenses against bacterial infections.Curr Opin Microbiol. 2013 Feb;16(1):23-31. doi: 10.1016/j.mib.2012.11.008. Epub 2013 Jan 11. Curr Opin Microbiol. 2013. PMID: 23318142 Free PMC article. Review.
Cited by
-
Post-translational regulation of inflammasomes.Cell Mol Immunol. 2017 Jan;14(1):65-79. doi: 10.1038/cmi.2016.29. Epub 2016 Jun 27. Cell Mol Immunol. 2017. PMID: 27345727 Free PMC article. Review.
-
Holding the inflammatory system in check: NLRs keep it cool.F1000Prime Rep. 2015 Feb 3;7:15. doi: 10.12703/P7-15. eCollection 2015. F1000Prime Rep. 2015. PMID: 25750733 Free PMC article. Review.
-
PANoptosis in cancer, the triangle of cell death.Cancer Med. 2023 Dec;12(24):22206-22223. doi: 10.1002/cam4.6803. Epub 2023 Dec 8. Cancer Med. 2023. PMID: 38069556 Free PMC article. Review.
-
Cell death programs in Yersinia immunity and pathogenesis.Front Cell Infect Microbiol. 2012 Nov 30;2:149. doi: 10.3389/fcimb.2012.00149. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23226685 Free PMC article. Review.
-
Bacterial secretion systems and regulation of inflammasome activation.J Leukoc Biol. 2017 Jan;101(1):165-181. doi: 10.1189/jlb.4MR0716-330R. Epub 2016 Nov 3. J Leukoc Biol. 2017. PMID: 27810946 Free PMC article. Review.
References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. - PubMed
-
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI064349/AI/NIAID NIH HHS/United States
- T32 AI095213/AI/NIAID NIH HHS/United States
- AI095213/AI/NIAID NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- AI64349/AI/NIAID NIH HHS/United States
- DK32520/DK/NIDDK NIH HHS/United States
- AI057159/AI/NIAID NIH HHS/United States
- R01 AI075318/AI/NIAID NIH HHS/United States
- AI075318/AI/NIAID NIH HHS/United States
- R01 AI083713/AI/NIAID NIH HHS/United States
- AI083713/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- AI057588/AI/NIAID NIH HHS/United States
- R01 AI057588/AI/NIAID NIH HHS/United States
- R56 AI075318/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

