BRET and Time-resolved FRET strategy to study GPCR oligomerization: from cell lines toward native tissues
- PMID: 22837753
- PMCID: PMC3401989
- DOI: 10.3389/fendo.2012.00092
BRET and Time-resolved FRET strategy to study GPCR oligomerization: from cell lines toward native tissues
Abstract
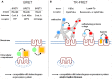
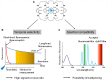
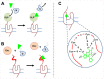
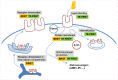
The concept of oligomerization of G protein-coupled receptor (GPCR) opens new perspectives regarding physiological function regulation. The capacity of one GPCR to modify its binding and coupling properties by interacting with a second one can be at the origin of regulations unsuspected two decades ago. Although the concept is interesting, its validation at a physiological level is challenging and probably explains why receptor oligomerization is still controversial. Demonstrating direct interactions between two proteins is not trivial since few techniques present a spatial resolution allowing this precision. Resonance energy transfer (RET) strategies are actually the most convenient ones. During the last two decades, bioluminescent resonance energy transfer and time-resolved fluorescence resonance energy transfer (TR-FRET) have been widely used since they exhibit high signal-to-noise ratio. Most of the experiments based on GPCR labeling have been performed in cell lines and it has been shown that all GPCRs have the propensity to form homo- or hetero-oligomers. However, whether these data can be extrapolated to GPCRs expressed in native tissues and explain receptor functioning in real life, remains an open question. Native tissues impose different constraints since GPCR sequences cannot be modified. Recently, a fluorescent ligand-based GPCR labeling strategy combined to a TR-FRET approach has been successfully used to prove the existence of GPCR oligomerization in native tissues. Although the RET-based strategies are generally quite simple to implement, precautions have to be taken before concluding to the absence or the existence of specific interactions between receptors. For example, one should exclude the possibility of collision of receptors diffusing throughout the membrane leading to a specific FRET signal. The advantages and the limits of different approaches will be reviewed and the consequent perspectives discussed.
Keywords: BRET; FRET; G protein-coupled receptor; fluorescence; fluorescent ligand; oligomer; time-resolved FRET.
Figures
Similar articles
-
Time resolved FRET strategy with fluorescent ligands to analyze receptor interactions in native tissues: application to GPCR oligomerization.Methods Mol Biol. 2011;746:373-87. doi: 10.1007/978-1-61779-126-0_21. Methods Mol Biol. 2011. PMID: 21607869
-
Time-Resolved FRET-Based Assays to Characterize G Protein-Coupled Receptor Hetero-oligomer Pharmacology.Methods Mol Biol. 2019;1947:151-168. doi: 10.1007/978-1-4939-9121-1_8. Methods Mol Biol. 2019. PMID: 30969415
-
Fluorescent ligands to investigate GPCR binding properties and oligomerization.Biochem Soc Trans. 2013 Feb 1;41(1):148-53. doi: 10.1042/BST20120237. Biochem Soc Trans. 2013. PMID: 23356275
-
Oligomerization of G protein-coupled receptors: biochemical and biophysical methods.Curr Med Chem. 2011;18(30):4606-34. doi: 10.2174/092986711797379285. Curr Med Chem. 2011. PMID: 21864280 Review.
-
Excitements and challenges in GPCR oligomerization: molecular insight from FRET.ACS Chem Neurosci. 2015 Jan 21;6(1):199-206. doi: 10.1021/cn500231d. Epub 2014 Nov 11. ACS Chem Neurosci. 2015. PMID: 25363209 Review.
Cited by
-
Structure-based network analysis of an evolved G protein-coupled receptor homodimer interface.Protein Sci. 2013 Jun;22(6):745-54. doi: 10.1002/pro.2258. Epub 2013 May 8. Protein Sci. 2013. PMID: 23553730 Free PMC article.
-
Cerebellar soluble mutant ataxin-3 level decreases during disease progression in Spinocerebellar Ataxia Type 3 mice.PLoS One. 2013 Apr 23;8(4):e62043. doi: 10.1371/journal.pone.0062043. Print 2013. PLoS One. 2013. PMID: 23626768 Free PMC article.
-
The evolving small-molecule fluorescent-conjugate toolbox for Class A GPCRs.Br J Pharmacol. 2014 Mar;171(5):1073-84. doi: 10.1111/bph.12265. Br J Pharmacol. 2014. PMID: 23734587 Free PMC article. Review.
-
Overcoming the Challenges of Detecting GPCR Oligomerization in the Brain.Curr Neuropharmacol. 2022;20(6):1035-1045. doi: 10.2174/1570159X19666211104145727. Curr Neuropharmacol. 2022. PMID: 34736381 Free PMC article.
-
G protein-coupled receptor-effector macromolecular membrane assemblies (GEMMAs).Pharmacol Ther. 2022 Mar;231:107977. doi: 10.1016/j.pharmthera.2021.107977. Epub 2021 Sep 1. Pharmacol Ther. 2022. PMID: 34480967 Free PMC article. Review.
References
-
- Achour L., Kamal M., Jockers R., Marullo S. (2011). Using quantitative BRET to assess G protein-coupled receptor homo- and heterodimerization. Methods Mol. Biol. 756 183–200 - PubMed
-
- Albizu L., Balestre M. N., Breton C., Pin J. P., Manning M., Mouillac B., Barberis C., Durroux T. (2006). Probing the existence of G protein-coupled receptor dimers by positive and negative ligand-dependent cooperative binding. Mol. Pharmacol. 70 1783–1791 - PubMed
-
- Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., Breton C., Rives M. L., Newman A., Javitch J., Trinquet E., Manning M., Pin J. P., Mouillac B., Durroux T. (2010). Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6 587–594 - PMC - PubMed
-
- Albizu L., Teppaz G., Seyer R., Bazin H., Ansanay H., Manning M., Mouillac B., Durroux T. (2007). Toward efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J. Med. Chem. 50 4976–4985 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

