Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury
- PMID: 22836271
- PMCID: PMC6703730
- DOI: 10.1523/JNEUROSCI.1498-12.2012
Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury
Abstract
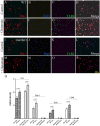
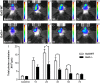
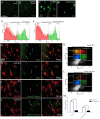
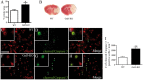
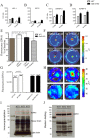
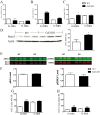
Growing evidence suggests that galectin-3 is involved in fine tuning of the inflammatory responses at the periphery, however, its role in injured brain is far less clear. Our previous work demonstrated upregulation and coexpression of galectin-3 and IGF-1 in a subset of activated/proliferating microglial cells after stroke. Here, we tested the hypothesis that galectin-3 plays a pivotal role in mediating injury-induced microglial activation and proliferation. By using a galectin-3 knock-out mouse (Gal-3KO), we demonstrated that targeted disruption of the galectin-3 gene significantly alters microglia activation and induces ∼4-fold decrease in microglia proliferation. Defective microglia activation/proliferation was further associated with significant increase in the size of ischemic lesion, ∼2-fold increase in the number of apoptotic neurons, and a marked deregulation of the IGF-1 levels. Next, our results revealed that contrary to WT cells, the Gal3-KO microglia failed to proliferate in response to IGF-1. Moreover, the IGF-1-mediated mitogenic microglia response was reduced by N-glycosylation inhibitor tunicamycine while coimmunoprecipitation experiments revealed galectin-3 binding to IGF-receptor 1 (R1), thus suggesting that interaction of galectin-3 with the N-linked glycans of receptors for growth factors is involved in IGF-R1 signaling. While the canonical IGF-1 signaling pathways were not affected, we observed an overexpression of IL-6 and SOCS3, suggesting an overactivation of JAK/STAT3, a shared signaling pathway for IGF-1/IL-6. Together, our findings suggest that galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury.
Figures

Similar articles
-
Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain.J Neurosci. 2007 Mar 7;27(10):2596-605. doi: 10.1523/JNEUROSCI.5360-06.2007. J Neurosci. 2007. PMID: 17344397 Free PMC article.
-
Delayed Galectin-3-Mediated Reprogramming of Microglia After Stroke is Protective.Mol Neurobiol. 2019 Sep;56(9):6371-6385. doi: 10.1007/s12035-019-1527-0. Epub 2019 Feb 23. Mol Neurobiol. 2019. PMID: 30798442
-
IGF-I and microglia/macrophage proliferation in the ischemic mouse brain.Glia. 2002 Jul;39(1):85-97. doi: 10.1002/glia.10081. Glia. 2002. PMID: 12112378
-
Galectin-3: mediator of microglia responses in injured brain.Drug Discov Today. 2018 Feb;23(2):375-381. doi: 10.1016/j.drudis.2017.11.004. Epub 2017 Nov 10. Drug Discov Today. 2018. PMID: 29133191 Review.
-
New roles of NCX in glial cells: activation of microglia in ischemia and differentiation of oligodendrocytes.Adv Exp Med Biol. 2013;961:307-16. doi: 10.1007/978-1-4614-4756-6_26. Adv Exp Med Biol. 2013. PMID: 23224890 Review.
Cited by
-
Microglia-mediated neuroinflammation and neuroplasticity after stroke.Front Cell Neurosci. 2022 Aug 16;16:980722. doi: 10.3389/fncel.2022.980722. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36052339 Free PMC article. Review.
-
Galectins-Potential Therapeutic Targets for Neurodegenerative Disorders.Int J Mol Sci. 2022 Sep 20;23(19):11012. doi: 10.3390/ijms231911012. Int J Mol Sci. 2022. PMID: 36232314 Free PMC article. Review.
-
Reformulating Pro-Oxidant Microglia in Neurodegeneration.J Clin Med. 2019 Oct 17;8(10):1719. doi: 10.3390/jcm8101719. J Clin Med. 2019. PMID: 31627485 Free PMC article. Review.
-
Mst1: Function and Mechanism in Brain and Myocardial Ischemia Reperfusion Injury.Curr Neuropharmacol. 2018;16(9):1358-1364. doi: 10.2174/1570159X16666180516095949. Curr Neuropharmacol. 2018. PMID: 29766810 Free PMC article. Review.
-
Invasion of phagocytic Galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair.Sci Adv. 2021 Aug 18;7(34):eabg2712. doi: 10.1126/sciadv.abg2712. Print 2021 Aug. Sci Adv. 2021. PMID: 34407943 Free PMC article.
References
-
- Abroun S, Ishikawa H, Tsuyama N, Liu S, Li FJ, Otsuyama K, Zheng X, Obata M, Kawano MM. Receptor synergy of interleukin-6 (IL-6) and insulin-like growth factor-I in myeloma cells that highly express IL-6 receptor alpha [corrected] Blood. 2004;103:2291–2298. - PubMed
-
- Alexandrova ML, Bochev PG. Oxidative stress during the chronic phase after stroke. Free Radic Biol Med. 2005;39:297–316. - PubMed
-
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. - PubMed
-
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
-
- Beaulieu JM, Kriz J, Julien JP. Induction of peripherin expression in subsets of brain neurons after lesion injury or cerebral ischemia. Brain Res. 2002;946:153–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
