Targeting MCL-1 sensitizes FLT3-ITD-positive leukemias to cytotoxic therapies
- PMID: 22829255
- PMCID: PMC3317524
- DOI: 10.1038/bcj.2012.5
Targeting MCL-1 sensitizes FLT3-ITD-positive leukemias to cytotoxic therapies
Abstract
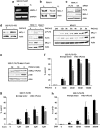
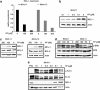
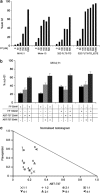
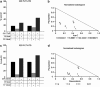
Patients suffering from acute myeloid leukemias (AML) bearing FMS-like tyrosine kinase-3-internal tandem duplications (FLT3-ITD) have poor outcomes following cytarabine- and anthracyclin-based induction therapy. To a major part this is attributed to drug resistance of FLT3-ITD-positive leukemic cells. Against this background, we have devised an antibody array approach to identify proteins, which are differentially expressed by hematopoietic cells in relation to activated FLT3 signaling. Selective upregulation of antiapoptotic myeloid cell leukemia-1 (MCL-1) was found in FLT3-ITD-positive cell lines and primary mononuclear cells from AML patients as compared with FLT3-wild-type controls. Upregulation of MCL-1 was dependent on FLT3 signaling as confirmed by its reversion upon pharmacological inhibition of FLT3 activity by the kinase inhibitor PKC412 as well as siRNA-mediated suppression of FLT3. Heterologously expressed MCL-1 substituted for FLT3 signaling by conferring resistance of hematopoietic cells to antileukemia drugs such as cytarabine and daunorubicin, and to the proapoptotic BH3 mimetic ABT-737. Conversely, suppression of endogenous MCL-1 by siRNA or by flavopiridol treatment sensitized FLT3-ITD-expressing hematopoietic cells to cytotoxic and targeted therapeutics. In conclusion, MCL-1 is an essential effector of FLT3-ITD-mediated drug resistance. Therapeutic targeting of MCL-1 is a promising strategy to overcome drug resistance in FLT3-ITD-positive AML.
Figures


Similar articles
-
FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation.Blood. 2009 Dec 3;114(24):5034-43. doi: 10.1182/blood-2008-12-196055. Epub 2009 Oct 6. Blood. 2009. PMID: 19808698 Free PMC article.
-
BH3-only protein Bim more critical than Puma in tyrosine kinase inhibitor-induced apoptosis of human leukemic cells and transduced hematopoietic progenitors carrying oncogenic FLT3.Blood. 2009 Mar 5;113(10):2302-11. doi: 10.1182/blood-2008-07-167023. Epub 2008 Dec 8. Blood. 2009. PMID: 19064725 Free PMC article.
-
Pim kinase inhibition sensitizes FLT3-ITD acute myeloid leukemia cells to topoisomerase 2 inhibitors through increased DNA damage and oxidative stress.Oncotarget. 2016 Jul 26;7(30):48280-48295. doi: 10.18632/oncotarget.10209. Oncotarget. 2016. PMID: 27374090 Free PMC article.
-
The role of small molecule Flt3 receptor protein-tyrosine kinase inhibitors in the treatment of Flt3-positive acute myelogenous leukemias.Pharmacol Res. 2020 May;155:104725. doi: 10.1016/j.phrs.2020.104725. Epub 2020 Feb 25. Pharmacol Res. 2020. PMID: 32109580 Review.
-
Targeted therapies in the treatment of adult acute myeloid leukemias: current status and future perspectives.Int J Hematol Oncol. 2016 Dec;5(4):143-164. doi: 10.2217/ijh-2016-0011. Epub 2017 Feb 7. Int J Hematol Oncol. 2016. PMID: 30302215 Free PMC article. Review.
Cited by
-
Acid ceramidase is upregulated in AML and represents a novel therapeutic target.Oncotarget. 2016 Dec 13;7(50):83208-83222. doi: 10.18632/oncotarget.13079. Oncotarget. 2016. PMID: 27825124 Free PMC article.
-
Venetoclax for AML: changing the treatment paradigm.Blood Adv. 2019 Dec 23;3(24):4326-4335. doi: 10.1182/bloodadvances.2019000937. Blood Adv. 2019. PMID: 31869416 Free PMC article.
-
Recent Updates in Venetoclax Combination Therapies in Pediatric Hematological Malignancies.Int J Mol Sci. 2023 Nov 24;24(23):16708. doi: 10.3390/ijms242316708. Int J Mol Sci. 2023. PMID: 38069030 Free PMC article. Review.
-
Pim Kinase Inhibitors Increase Gilteritinib Cytotoxicity in FLT3-ITD Acute Myeloid Leukemia Through GSK-3β Activation and c-Myc and Mcl-1 Proteasomal Degradation.Cancer Res Commun. 2024 Feb 16;4(2):431-445. doi: 10.1158/2767-9764.CRC-23-0379. Cancer Res Commun. 2024. PMID: 38284896 Free PMC article.
-
The E3 ubiquitin ligase TRAF6 inhibits LPS-induced AKT activation in FLT3-ITD-positive MV4-11 AML cells.J Cancer Res Clin Oncol. 2013 Apr;139(4):605-15. doi: 10.1007/s00432-012-1362-4. Epub 2012 Dec 21. J Cancer Res Clin Oncol. 2013. PMID: 23263202
References
-
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. - PubMed
-
- Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. - PubMed
-
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. - PubMed
-
- Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. - PubMed
-
- Kindler T, Breitenbuecher F, Kasper S, Estey E, Giles F, Feldman E, et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML) Blood. 2005;105:335–340. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

