Epigenetic control of cell cycle-dependent histone gene expression is a principal component of the abbreviated pluripotent cell cycle
- PMID: 22826438
- PMCID: PMC3457543
- DOI: 10.1128/MCB.00736-12
Epigenetic control of cell cycle-dependent histone gene expression is a principal component of the abbreviated pluripotent cell cycle
Abstract
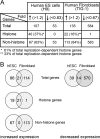
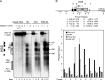
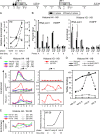
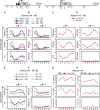
Self-renewal of human pluripotent embryonic stem cells proceeds via an abbreviated cell cycle with a shortened G(1) phase. We examined which genes are modulated in this abbreviated period and the epigenetic mechanisms that control their expression. Accelerated upregulation of genes encoding histone proteins that support DNA replication is the most prominent gene regulatory program at the G(1)/S-phase transition in pluripotent cells. Expedited expression of histone genes is mediated by a unique chromatin architecture reflected by major nuclease hypersensitive sites, atypical distribution of epigenetic histone marks, and a region devoid of histone octamers. We observed remarkable differences in chromatin structure--hypersensitivity and histone protein modifications--between human embryonic stem (hES) and normal diploid cells. Cell cycle-dependent transcription factor binding permits dynamic three-dimensional interactions between transcript initiating and processing factors at 5' and 3' regions of the gene. Thus, progression through the abbreviated G(1) phase involves cell cycle stage-specific chromatin-remodeling events and rapid assembly of subnuclear microenvironments that activate histone gene transcription to promote nucleosomal packaging of newly replicated DNA during stem cell renewal.
Figures

Similar articles
-
Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells.J Cell Physiol. 2007 Feb;210(2):517-26. doi: 10.1002/jcp.20903. J Cell Physiol. 2007. PMID: 17096384
-
Reprogramming the pluripotent cell cycle: restoration of an abbreviated G1 phase in human induced pluripotent stem (iPS) cells.J Cell Physiol. 2011 May;226(5):1149-56. doi: 10.1002/jcp.22440. J Cell Physiol. 2011. PMID: 20945438 Free PMC article.
-
Staged assembly of histone gene expression machinery at subnuclear foci in the abbreviated cell cycle of human embryonic stem cells.Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):16964-9. doi: 10.1073/pnas.0809273105. Epub 2008 Oct 28. Proc Natl Acad Sci U S A. 2008. PMID: 18957539 Free PMC article.
-
Cell cycle dependent phosphorylation and subnuclear organization of the histone gene regulator p220(NPAT) in human embryonic stem cells.J Cell Physiol. 2007 Oct;213(1):9-17. doi: 10.1002/jcp.21119. J Cell Physiol. 2007. PMID: 17520687 Review.
-
Chromatin regulation and dynamics in stem cells.Curr Top Dev Biol. 2020;138:1-71. doi: 10.1016/bs.ctdb.2019.11.002. Epub 2019 Dec 30. Curr Top Dev Biol. 2020. PMID: 32220294 Free PMC article. Review.
Cited by
-
Cell cycle gene expression networks discovered using systems biology: Significance in carcinogenesis.J Cell Physiol. 2015 Oct;230(10):2533-42. doi: 10.1002/jcp.24990. J Cell Physiol. 2015. PMID: 25808367 Free PMC article.
-
Lessons learned about human stem cell responses to ionizing radiation exposures: a long road still ahead of us.Int J Mol Sci. 2013 Jul 29;14(8):15695-723. doi: 10.3390/ijms140815695. Int J Mol Sci. 2013. PMID: 23899786 Free PMC article. Review.
-
Spatiotemporal Epigenetic Control of the Histone Gene Chromatin Landscape during the Cell Cycle.Crit Rev Eukaryot Gene Expr. 2023;33(3):85-97. doi: 10.1615/CritRevEukaryotGeneExpr.2022046190. Crit Rev Eukaryot Gene Expr. 2023. PMID: 37017672 Free PMC article.
-
Fidelity of histone gene regulation is obligatory for genome replication and stability.Mol Cell Biol. 2014 Jul;34(14):2650-9. doi: 10.1128/MCB.01567-13. Mol Cell Biol. 2014. PMID: 24797072 Free PMC article.
-
Genome-wide identification of histone methylation (H3K9me2) and acetylation (H4K12ac) marks in two ecotypes of switchgrass (Panicum virgatum L.).BMC Genomics. 2019 Aug 22;20(1):667. doi: 10.1186/s12864-019-6038-x. BMC Genomics. 2019. PMID: 31438854 Free PMC article.
References
-
- Ahmed K, et al. 2010. Global chromatin architecture reflects pluripotency and lineage commitment in the early mouse embryo. PLoS One 5:e10531 doi:10.1371/journal.pone.0010531 - DOI - PMC - PubMed
-
- Becker KA, et al. 2006. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J. Cell. Physiol. 209:883–893 - PubMed
-
- Becker KA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. 2007. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J. Cell. Physiol. 210:517–526 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
