A Rho GTPase signal treadmill backs a contractile array
- PMID: 22819338
- PMCID: PMC3549422
- DOI: 10.1016/j.devcel.2012.05.025
A Rho GTPase signal treadmill backs a contractile array
Abstract
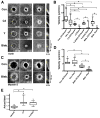
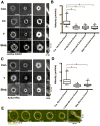
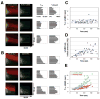
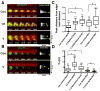
Contractile arrays of actin filaments (F-actin) and myosin-2 power diverse biological processes. Contractile array formation is stimulated by the Rho GTPases Rho and Cdc42; after assembly, array movement is thought to result from contraction itself. Contractile array movement and GTPase activity were analyzed during cellular wound repair, in which arrays close in association with zones of Rho and Cdc42 activity. Remarkably, contraction suppression prevents translocation of F-actin and myosin-2 without preventing array or zone closure. Closure is driven by an underlying "signal treadmill" in which the GTPases are preferentially activated at the leading edges and preferentially lost from the trailing edges of their zones. Treadmill organization requires myosin-2-powered contraction and F-actin turnover. Thus, directional gradients in Rho GTPase turnover impart directional information to contractile arrays, and proper functioning of these gradients is dependent on both contraction and F-actin turnover.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Wound healing: GTPases flux their muscles.Dev Cell. 2012 Aug 14;23(2):236-8. doi: 10.1016/j.devcel.2012.07.017. Dev Cell. 2012. PMID: 22898773
Similar articles
-
Rho kinase collaborates with p21-activated kinase to regulate actin polymerization and contraction in airway smooth muscle.J Physiol. 2018 Aug;596(16):3617-3635. doi: 10.1113/JP275751. Epub 2018 Jun 24. J Physiol. 2018. PMID: 29746010 Free PMC article.
-
Rho GTPase activity zones and transient contractile arrays.Bioessays. 2006 Oct;28(10):983-93. doi: 10.1002/bies.20477. Bioessays. 2006. PMID: 16998826 Free PMC article. Review.
-
Modeling the roles of protein kinase Cβ and η in single-cell wound repair.Mol Biol Cell. 2015 Nov 5;26(22):4100-8. doi: 10.1091/mbc.E15-06-0383. Epub 2015 Aug 26. Mol Biol Cell. 2015. PMID: 26310444 Free PMC article.
-
Control of local Rho GTPase crosstalk by Abr.Curr Biol. 2011 Feb 22;21(4):270-7. doi: 10.1016/j.cub.2011.01.014. Epub 2011 Feb 3. Curr Biol. 2011. PMID: 21295482 Free PMC article.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
-
Traveling waves in actin dynamics and cell motility.Curr Opin Cell Biol. 2013 Feb;25(1):107-15. doi: 10.1016/j.ceb.2012.08.012. Epub 2012 Sep 15. Curr Opin Cell Biol. 2013. PMID: 22985541 Free PMC article. Review.
-
Plasma membrane damage limits replicative lifespan in yeast and induces premature senescence in human fibroblasts.Nat Aging. 2024 Mar;4(3):319-335. doi: 10.1038/s43587-024-00575-6. Epub 2024 Feb 22. Nat Aging. 2024. PMID: 38388781 Free PMC article.
-
Pattern formation of Rho GTPases in single cell wound healing.Mol Biol Cell. 2013 Feb;24(3):421-32. doi: 10.1091/mbc.E12-08-0634. Epub 2012 Dec 21. Mol Biol Cell. 2013. PMID: 23264464 Free PMC article.
-
An Overview of Cell Membrane Perforation and Resealing Mechanisms for Localized Drug Delivery.Pharmaceutics. 2022 Apr 18;14(4):886. doi: 10.3390/pharmaceutics14040886. Pharmaceutics. 2022. PMID: 35456718 Free PMC article. Review.
-
Coordinated efforts of different actin filament populations are needed for optimal cell wound repair.Mol Biol Cell. 2023 Mar 1;34(3):ar15. doi: 10.1091/mbc.E22-05-0155. Epub 2023 Jan 4. Mol Biol Cell. 2023. PMID: 36598808 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

