TNFRSF25 agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions
- PMID: 22811539
- PMCID: PMC3457251
- DOI: 10.1128/JVI.01391-12
TNFRSF25 agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions
Abstract
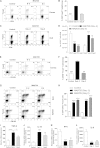
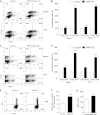
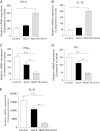
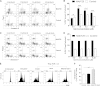
Ocular infection with herpes simplex virus 1 (HSV-1) results in a chronic immunoinflamammtory reaction in the cornea, which is primarily orchestrated by CD4(+) T cells. Hence, targeting proinflammatory CD4(+) T cells or increasing the representation of cells that regulate their function is a relevant therapeutic strategy. In this report, we demonstrate that effective therapeutic control can be achieved using a combination of approaches under circumstances where monotherapy is ineffective. We use a convenient and highly effective monoclonal antibody (MAb) approach with MAbT25 to expand cells that express the tumor necrosis factor receptor superfamily member 25 (TNFRSF25). In naïve animals, these are predominantly cells that are Foxp3-positive regulatory T cells. MAbT25 treatment before or at the time of initial HSV infection was an effective means of reducing the severity of subsequent stromal keratitis lesions. However, MAbT25 treatment was not effective if given 6 days after infection since it expanded proinflammatory effector T cells, which also express TNFRSF25. Therefore, the MAbT25 procedure was combined with galectin-9 (Gal-9), an approach that compromises the activity of T cells involved in tissue damage. The combination therapy provided highly effective lesion control over that achieved by treatment with one of them. The beneficial outcome of the combination therapy was attributed to the expansion of the regulatory T cell population that additionally expressed activation markers such as CD103 needed to access inflammatory sites. Additionally, there was a marked reduction of CD4(+) gamma interferon-producing effector T cells responsible for orchestrating the tissue damage. The approach that we describe has potential application to control a wide range of inflammatory diseases, in addition to stromal keratitis, an important cause of human blindness.
Figures





Similar articles
-
Azacytidine Treatment Inhibits the Progression of Herpes Stromal Keratitis by Enhancing Regulatory T Cell Function.J Virol. 2017 Mar 13;91(7):e02367-16. doi: 10.1128/JVI.02367-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28100624 Free PMC article.
-
In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions.J Virol. 2008 Jul;82(14):6838-51. doi: 10.1128/JVI.00697-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480441 Free PMC article.
-
Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators.J Immunol. 2009 Mar 1;182(5):3191-201. doi: 10.4049/jimmunol.0803673. J Immunol. 2009. PMID: 19234217 Free PMC article.
-
The Role of T Cells in Herpes Stromal Keratitis.Front Immunol. 2019 Mar 19;10:512. doi: 10.3389/fimmu.2019.00512. eCollection 2019. Front Immunol. 2019. PMID: 30941142 Free PMC article. Review.
-
Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators.Front Immunol. 2020 May 13;11:766. doi: 10.3389/fimmu.2020.00766. eCollection 2020. Front Immunol. 2020. PMID: 32477330 Free PMC article. Review.
Cited by
-
Restriction of Human Cytomegalovirus Infection by Galectin-9.J Virol. 2019 Jan 17;93(3):e01746-18. doi: 10.1128/JVI.01746-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30487283 Free PMC article.
-
IL-17A-mediated protection against Acanthamoeba keratitis.J Immunol. 2015 Jan 15;194(2):650-63. doi: 10.4049/jimmunol.1302707. Epub 2014 Dec 10. J Immunol. 2015. PMID: 25505284 Free PMC article.
-
A Synthetic Tetramer of Galectin-1 and Galectin-3 Amplifies Pro-apoptotic Signaling by Integrating the Activity of Both Galectins.Front Chem. 2020 Jan 10;7:898. doi: 10.3389/fchem.2019.00898. eCollection 2019. Front Chem. 2020. PMID: 31998689 Free PMC article.
-
Role of regulatory T cells during virus infection.Immunol Rev. 2013 Sep;255(1):182-96. doi: 10.1111/imr.12085. Immunol Rev. 2013. PMID: 23947355 Free PMC article. Review.
-
Regulatory T Cell-Mediated Suppression of Inflammation Induced by DR3 Signaling Is Dependent on Galectin-9.J Immunol. 2017 Oct 15;199(8):2721-2728. doi: 10.4049/jimmunol.1700575. Epub 2017 Sep 6. J Immunol. 2017. PMID: 28877989 Free PMC article.
References
-
- Anderson AC, Anderson DE. 2006. TIM-3 in autoimmunity. Curr. Opin. Immunol. 18:665–669 - PubMed
-
- Blaskó A, Fajka-Boja R, Ion G, Monostori E. 2011. How does it act when soluble? Critical evaluation of mechanism of galectin-1 induced T-cell apoptosis. Acta Biol. Hung. 62:106–111 - PubMed
-
- Chinnaiyan AM, et al. 1996. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 274:990–992 - PubMed
-
- Crawford A, Wherry EJ. 2007. Inhibitory receptors: whose side are they on? Nat. Immunol. 8:1201–1203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

