Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system
- PMID: 22804625
- PMCID: PMC4085275
- DOI: 10.1021/nn3015008
Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system
Abstract
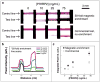
There is a need for simple yet robust biomarker and antigen purification and enrichment strategies that are compatible with current rapid diagnostic modalities. Here, a stimuli-responsive nanoparticle system is presented for multiplexed magneto-enrichment and non-instrumented lateral flow strip detection of model antigens from spiked pooled plasma. The integrated reagent system allows purification and enrichment of the gold-labeled biomarker half-sandwich that can be applied directly to lateral flow test strips. A linear diblock copolymer with a thermally responsive poly(N-isopropylacrylamide) (pNIPAm) segment and a gold-binding block composed of NIPAm-co-N,N-dimethylaminoethylacrylamide was prepared by reversible addition-fragmentation chain transfer polymerization. The diblock copolymer was used to functionalize gold nanoparticles (AuNPs), with subsequent bioconjugation to yield thermally responsive pNIPAm-AuNPs that were co-decorated with streptavidin. These AuNPs efficiently complexed biotinylated capture antibody reagents that were bound to picomolar quantities of pan-aldolase and Plasmodium falciparum histidine-rich protein 2 (PfHRP2) in spiked pooled plasma samples. The gold-labeled biomarker half-sandwich was then purified and enriched using 10 nm thermally responsive magnetic nanoparticles that were similarly decorated with pNIPAm. When a thermal stimulus was applied in conjunction with a magnetic field, coaggregation of the AuNP half-sandwiches with the pNIPAm-coated iron oxide nanoparticles created large aggregates that were efficiently magnetophoresed and separated from bulk serum. The purified biomarkers from a spiked pooled plasma sample could be concentrated 50-fold into a small volume and applied directly to a commercial multiplexed lateral flow strip to dramatically improve the signal-to-noise ratio and test sensitivity.
Figures





Similar articles
-
Improving lateral-flow immunoassay (LFIA) diagnostics via biomarker enrichment for mHealth.Methods Mol Biol. 2015;1256:71-84. doi: 10.1007/978-1-4939-2172-0_5. Methods Mol Biol. 2015. PMID: 25626532
-
Mixed stimuli-responsive magnetic and gold nanoparticle system for rapid purification, enrichment, and detection of biomarkers.Bioconjug Chem. 2010 Dec 15;21(12):2197-204. doi: 10.1021/bc100180q. Epub 2010 Nov 11. Bioconjug Chem. 2010. PMID: 21070026 Free PMC article.
-
Simple fluidic system for purifying and concentrating diagnostic biomarkers using stimuli-responsive antibody conjugates and membranes.Bioconjug Chem. 2010 Oct 20;21(10):1820-6. doi: 10.1021/bc100169y. Bioconjug Chem. 2010. PMID: 20845976 Free PMC article.
-
Functional Micro-/Nanomaterials for Multiplexed Biodetection.Adv Mater. 2021 Jul;33(30):e2004734. doi: 10.1002/adma.202004734. Epub 2021 Jun 17. Adv Mater. 2021. PMID: 34137090 Review.
-
Immunomagnetic nanoparticle-based assays for detection of biomarkers.Int J Nanomedicine. 2013;8:4543-52. doi: 10.2147/IJN.S51893. Epub 2013 Nov 22. Int J Nanomedicine. 2013. PMID: 24285924 Free PMC article. Review.
Cited by
-
A Multiple-Stimuli-Responsive Amphiphilic Copolymer for Antifouling and Antibacterial Functionality via a "Resistance-Kill-Release" Mechanism.Molecules. 2022 Aug 9;27(16):5059. doi: 10.3390/molecules27165059. Molecules. 2022. PMID: 36014312 Free PMC article.
-
Metallic Nanoparticles and Core-Shell Nanosystems in the Treatment, Diagnosis, and Prevention of Parasitic Diseases.Pathogens. 2023 Jun 17;12(6):838. doi: 10.3390/pathogens12060838. Pathogens. 2023. PMID: 37375528 Free PMC article. Review.
-
Acoustofluidic centrifuge for nanoparticle enrichment and separation.Sci Adv. 2021 Jan 1;7(1):eabc0467. doi: 10.1126/sciadv.abc0467. Print 2021 Jan. Sci Adv. 2021. PMID: 33523836 Free PMC article.
-
An ultrasensitive NIR-IIa' fluorescence-based multiplex immunochromatographic strip test platform for antibiotic residues detection in milk samples.J Adv Res. 2023 Aug;50:25-34. doi: 10.1016/j.jare.2022.10.008. Epub 2022 Oct 22. J Adv Res. 2023. PMID: 36280143 Free PMC article.
-
Phenylboronic acid-functionalized magnetic nanoparticles for one-step saccharides enrichment and mass spectrometry analysis.Biophys Rep. 2015;1:61-70. doi: 10.1007/s41048-015-0002-3. Epub 2015 Jul 14. Biophys Rep. 2015. PMID: 26942220 Free PMC article.
References
-
- Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral Flow (Immuno) Assay: Its Strengths, Weaknesses, Opportunities and Threats. A Literature Survey. Anal Bioanal Chem. 2009;393:569–582. - PubMed
-
- Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, Hoffman SL. Diagnosis of Malaria by Detection of Plasmodium-Falciparum Hrp-2 Antigen with a Rapid Dipstick Antigen-Capture Assay. Lancet. 1994;343:564–568. - PubMed
-
- Bell D, Peeling RW. Evaluation of Rapid Diagnostic Tests: Malaria. Nat Rev Microbiol. 2006:S34–S40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

