Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children
- PMID: 22802243
- PMCID: PMC3421858
- DOI: 10.1128/AAC.06446-11
Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children
Erratum in
- Antimicrob Agents Chemother. 2012 Oct;56(10):5431
Abstract
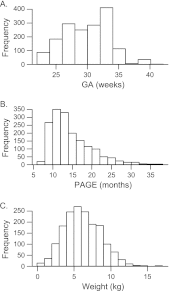
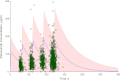
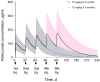
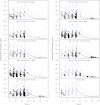
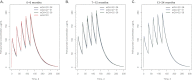
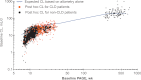
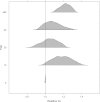
Although it has been on the market for over a decade, confusion remains regarding the pharmacokinetics (PK) and optimal dosing of palivizumab, a humanized IgG1κ monoclonal antibody indicated for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients at high risk of RSV disease. The objectives of this analysis were to characterize the population PK of palivizumab in adults and children using nonlinear mixed-effect modeling, quantify the effects of individual covariates on variability in palivizumab disposition, and compare palivizumab exposures for various dosing scenarios. Palivizumab PK data from 22 clinical studies were used for model development. The model was developed using a two-stage approach: (i) a 2-compartment model with first-order absorption after intramuscular administration was fitted to adult data, and (ii) the same structural model was fitted to the sparse pediatric data using the NONMEM $PRIOR subroutine, with informative priors obtained from the adult analysis. Body weight and an age descriptor that combines gestational age and postnatal age (PAGE) using an asymptotic-exponential model best described palivizumab clearance in pediatric patients. Palivizumab clearance increased slightly from 10.2 ml/day to 11.9 ml/day as a function of PAGE ranging from 7 to 18 months. Covariate analysis indicated a 20% higher clearance in children with chronic lung disease and in children with antidrug antibody titer values of ≥80. These covariates did not substantially explain interindividual variability. In the label-indicated pediatric population, body weight was the primary demographic factor affecting palivizumab PK. Body weight-based dosing of 15 mg/kg yields similar palivizumab concentrations in children of different gestational and postnatal ages. Simulations demonstrated that there was little difference in palivizumab PK between healthy term and premature infants. Simulations also demonstrated that the 5 monthly palivizumab doses of 15 mg/kg, consistent with the label and studied in two randomized, clinical trials, provided greater and more prolonged palivizumab exposure than did an abbreviated dosing regimen of 3 monthly doses.
Figures
Similar articles
-
Three monthly doses of palivizumab are not adequate for 5-month protection: a population pharmacokinetic analysis.Pulm Pharmacol Ther. 2013 Dec;26(6):666-71. doi: 10.1016/j.pupt.2013.03.007. Epub 2013 Mar 19. Pulm Pharmacol Ther. 2013. PMID: 23523663
-
Survey of pediatric ward hospitalization due to respiratory syncytial virus infection after the introduction of palivizumab to high-risk infants in Japan.Pediatr Int. 2011 Jun;53(3):368-73. doi: 10.1111/j.1442-200X.2010.03249.x. Pediatr Int. 2011. PMID: 20854284 Clinical Trial.
-
A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season.BMC Pediatr. 2010 Jun 3;10:38. doi: 10.1186/1471-2431-10-38. BMC Pediatr. 2010. PMID: 20525274 Free PMC article. Clinical Trial.
-
The use of humanized monoclonal antibodies for the prevention of respiratory syncytial virus infection.Clin Dev Immunol. 2013;2013:359683. doi: 10.1155/2013/359683. Epub 2013 Jun 11. Clin Dev Immunol. 2013. PMID: 23840240 Free PMC article. Review.
-
Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses.Health Technol Assess. 2011 Jan;15(5):iii-iv, 1-124. doi: 10.3310/hta15050. Health Technol Assess. 2011. PMID: 21281564 Free PMC article. Review.
Cited by
-
Minimal Physiologically-based Pharmacokinetic Model to Investigate the Effect of Charge on the Pharmacokinetics of Humanized anti-HCV-E2 IgG Antibodies in Sprague-Dawley Rats.Pharm Res. 2022 Mar;39(3):481-496. doi: 10.1007/s11095-022-03204-2. Epub 2022 Mar 4. Pharm Res. 2022. PMID: 35246757
-
Opportunities and Challenges for PBPK Model of mAbs in Paediatrics and Pregnancy.AAPS J. 2022 Jun 1;24(4):72. doi: 10.1208/s12248-022-00722-0. AAPS J. 2022. PMID: 35650328 Review.
-
Revised recommendations concerning palivizumab prophylaxis for respiratory syncytial virus (RSV).Ital J Pediatr. 2015 Dec 15;41:97. doi: 10.1186/s13052-015-0203-x. Ital J Pediatr. 2015. PMID: 26670908 Free PMC article. Review.
-
Pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of monoclonal antibodies in children.Clin Pharmacokinet. 2015 Jan;54(1):35-80. doi: 10.1007/s40262-014-0208-4. Clin Pharmacokinet. 2015. PMID: 25516414 Review.
-
Potential Sources of Inter-Subject Variability in Monoclonal Antibody Pharmacokinetics.Clin Pharmacokinet. 2016 Jul;55(7):789-805. doi: 10.1007/s40262-015-0361-4. Clin Pharmacokinet. 2016. PMID: 26818483 Review.
References
-
- Anderson BJ, Allegaert K, Holford NH. 2006. Population clinical pharmacology of children: modelling covariate effects. Eur. J. Pediatr. 165:819–829 - PubMed
-
- Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303–332 - PubMed
-
- Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24:25–36 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

