Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion
- PMID: 22801410
- PMCID: PMC3475769
- DOI: 10.1038/mp.2012.97
Pathogenic disruption of DISC1-serine racemase binding elicits schizophrenia-like behavior via D-serine depletion
Abstract
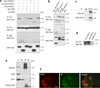
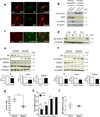
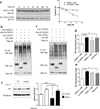
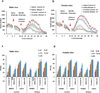
Perturbation of Disrupted-In-Schizophrenia-1 (DISC1) and D-serine/NMDA receptor hypofunction have both been implicated in the pathophysiology of schizophrenia and other psychiatric disorders. In the present study, we demonstrate that these two pathways intersect with behavioral consequences. DISC1 binds to and stabilizes serine racemase (SR), the enzyme that generates D-serine, an endogenous co-agonist of the NMDA receptor. Mutant DISC1 fails to bind to SR, facilitating ubiquitination and degradation of SR and a decrease in D-serine production. To elucidate DISC1-SR interactions in vivo, we generated a mouse model of selective and inducible expression of mutant DISC1 in astrocytes, the main source of D-serine in the brain. Expression of mutant DISC1 downregulates endogenous DISC1 and decreases protein but not mRNA levels of SR, resulting in diminished production of D-serine. In contrast, mutant DISC1 does not alter levels of ALDH1L1, connexins, GLT-1 or binding partners of DISC1 and SR, LIS1 or PICK1. Adult male and female mice with lifelong expression of mutant DISC1 exhibit behavioral abnormalities consistent with hypofunction of NMDA neurotransmission. Specifically, mutant mice display greater responses to an NMDA antagonist, MK-801, in open field and pre-pulse inhibition of the acoustic startle tests and are significantly more sensitive to the ameliorative effects of D-serine. These findings support a model wherein mutant DISC1 leads to SR degradation via dominant negative effects, resulting in D-serine deficiency that diminishes NMDA neurotransmission thus linking DISC1 and NMDA pathophysiological mechanisms in mental illness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Neuronal serine racemase associates with Disrupted-In-Schizophrenia-1 and DISC1 agglomerates: Implications for schizophrenia.Neurosci Lett. 2019 Jan 23;692:107-114. doi: 10.1016/j.neulet.2018.10.055. Epub 2018 Nov 1. Neurosci Lett. 2019. PMID: 30391323 Free PMC article.
-
DISC1 in Astrocytes Influences Adult Neurogenesis and Hippocampus-Dependent Behaviors in Mice.Neuropsychopharmacology. 2017 Oct;42(11):2242-2251. doi: 10.1038/npp.2017.129. Epub 2017 Jun 20. Neuropsychopharmacology. 2017. PMID: 28631721 Free PMC article.
-
Mutant disrupted-in-schizophrenia 1 in astrocytes: focus on glutamate metabolism.J Neurosci Res. 2014 Dec;92(12):1659-68. doi: 10.1002/jnr.23459. Epub 2014 Aug 8. J Neurosci Res. 2014. PMID: 25131692 Free PMC article.
-
The Role of Serine Racemase in the Pathophysiology of Brain Disorders.Adv Pharmacol. 2018;82:35-56. doi: 10.1016/bs.apha.2017.10.002. Epub 2017 Nov 29. Adv Pharmacol. 2018. PMID: 29413527 Free PMC article. Review.
-
Inhibition of human serine racemase, an emerging target for medicinal chemistry.Curr Drug Targets. 2011 Jun;12(7):1037-55. doi: 10.2174/138945011795677755. Curr Drug Targets. 2011. PMID: 21291385 Review.
Cited by
-
Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system.Nat Commun. 2015 Apr 9;6:6761. doi: 10.1038/ncomms7761. Nat Commun. 2015. PMID: 25854456 Free PMC article.
-
Astrocytes in schizophrenia.Brain Neurosci Adv. 2021 Apr 27;5:23982128211009148. doi: 10.1177/23982128211009148. eCollection 2021 Jan-Dec. Brain Neurosci Adv. 2021. PMID: 33997293 Free PMC article. Review.
-
Human Serine Racemase: Key Residues/Active Site Motifs and Their Relation to Enzyme Function.Front Mol Biosci. 2019 Mar 13;6:8. doi: 10.3389/fmolb.2019.00008. eCollection 2019. Front Mol Biosci. 2019. PMID: 30918891 Free PMC article. Review.
-
Targeting glia cells: novel perspectives for the treatment of neuropsychiatric diseases.Curr Neuropharmacol. 2013 Mar;11(2):171-85. doi: 10.2174/1570159X11311020004. Curr Neuropharmacol. 2013. PMID: 23997752 Free PMC article.
-
d-Serine administration affects nitric oxide synthase 1 adaptor protein and DISC1 expression in sex-specific manner.Mol Cell Neurosci. 2018 Jun;89:20-32. doi: 10.1016/j.mcn.2018.03.011. Epub 2018 Mar 27. Mol Cell Neurosci. 2018. PMID: 29601869 Free PMC article.
References
-
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. The American journal of psychiatry. 2001;158(9):1367–1377. - PubMed
-
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. The American journal of psychiatry. 1991;148(10):1301–1308. - PubMed
-
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 MH085226/MH/NIMH NIH HHS/United States
- R37 MH018501/MH/NIMH NIH HHS/United States
- MH084020/MH/NIMH NIH HHS/United States
- RC1 MH088753/MH/NIMH NIH HHS/United States
- R01 MH092443/MH/NIMH NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- MH083728/MH/NIMH NIH HHS/United States
- P50 MH094268/MH/NIMH NIH HHS/United States
- P50 MH084020/MH/NIMH NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- R01 MH083728/MH/NIMH NIH HHS/United States
- MH18501/MH/NIMH NIH HHS/United States
- R01 MH018501/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

