NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells
- PMID: 22798671
- PMCID: PMC3411873
- DOI: 10.4049/jimmunol.1200584
NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells
Abstract
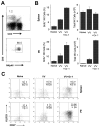
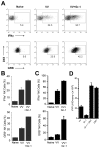
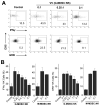
NK cells are critical for the innate immune control of poxviral infections. Previous studies have shown that NK cells are efficiently activated in response to infection with vaccinia virus (VV), the most studied member of the poxvirus family. However, it remains unknown whether the activation of NK cells in response to VV infection is tightly regulated. In this study, we showed that myeloid-derived suppressor cells (MDSCs) rapidly accumulated at the site of VV infection. In vivo depletion of MDSCs led to enhanced NK cell proliferation, activation, and function in response to VV infection. This was accompanied by an increase in mortality and systemic IFN-γ production. We further demonstrated that the granulocytic-MDSC (G-MDSC) subset was responsible for the suppression on NK cells and that this suppression was mediated by reactive oxygen species. These results indicate that G-MDSCs can negatively regulate NK cell activation and function in response to VV infection and suggest that manipulation of G-MDSCs could represent an attractive strategy for regulating NK cell activities for potential therapeutic benefits.
Figures


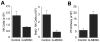

Similar articles
-
Monocytic myeloid-derived suppressor cells regulate T-cell responses against vaccinia virus.Eur J Immunol. 2017 Jun;47(6):1022-1031. doi: 10.1002/eji.201646797. Epub 2017 May 2. Eur J Immunol. 2017. PMID: 28383204 Free PMC article.
-
Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection.PLoS Pathog. 2010 Mar 12;6(3):e1000811. doi: 10.1371/journal.ppat.1000811. PLoS Pathog. 2010. PMID: 20300608 Free PMC article.
-
Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer.J Virol. 2012 Dec;86(24):13689-96. doi: 10.1128/JVI.01595-12. Epub 2012 Oct 10. J Virol. 2012. PMID: 23055553 Free PMC article.
-
Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression.Front Immunol. 2019 Apr 18;10:771. doi: 10.3389/fimmu.2019.00771. eCollection 2019. Front Immunol. 2019. PMID: 31057536 Free PMC article. Review.
-
Antigen specificity of immune suppression by myeloid-derived suppressor cells.J Leukoc Biol. 2011 Jul;90(1):31-6. doi: 10.1189/jlb.0111021. Epub 2011 Apr 12. J Leukoc Biol. 2011. PMID: 21486906 Review.
Cited by
-
Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences.Cell Mol Life Sci. 2013 Oct;70(20):3813-27. doi: 10.1007/s00018-013-1286-4. Epub 2013 Feb 20. Cell Mol Life Sci. 2013. PMID: 23423530 Free PMC article. Review.
-
Metastasis-associated macrophages constrain antitumor capability of natural killer cells in the metastatic site at least partially by membrane bound transforming growth factor β.J Immunother Cancer. 2021 Jan;9(1):e001740. doi: 10.1136/jitc-2020-001740. J Immunother Cancer. 2021. PMID: 33472858 Free PMC article.
-
Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms.Br J Cancer. 2014 Jan 7;110(1):83-93. doi: 10.1038/bjc.2013.695. Epub 2013 Nov 26. Br J Cancer. 2014. PMID: 24281006 Free PMC article.
-
Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment.Mol Ther. 2021 May 5;29(5):1782-1793. doi: 10.1016/j.ymthe.2020.12.024. Epub 2020 Dec 19. Mol Ther. 2021. PMID: 33348052 Free PMC article.
-
Myeloid-derived suppressor cells: the dark knight or the joker in viral infections?Immunol Rev. 2013 Sep;255(1):210-21. doi: 10.1111/imr.12084. Immunol Rev. 2013. PMID: 23947357 Free PMC article. Review.
References
-
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. - PubMed
-
- Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. - PubMed
-
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. - PubMed
-
- Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. - PubMed
-
- Natuk RJ, Welsh RM. Accumulation and chemotaxis of natural killer/large granular lymphocytes at sites of virus replication. J Immunol. 1987;138:877–883. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

