Autophagy in the regulation of pathogen replication and adaptive immunity
- PMID: 22796170
- PMCID: PMC3461100
- DOI: 10.1016/j.it.2012.06.003
Autophagy in the regulation of pathogen replication and adaptive immunity
Abstract
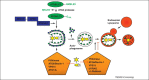
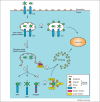
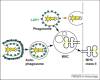
Autophagy is an evolutionarily conserved homeostatic process by which cells deliver cytoplasmic material for degradation into lysosomes. Autophagy may have evolved as a nutrient-providing homeostatic pathway induced upon starvation, but with the acquisition of cargo receptors, autophagy has become an important cellular defence mechanism as well as a generator of antigenic peptides for major histocompatibility complex (MHC) presentation. We propose that autophagy efficiently protects against microbes encountering the cytosolic environment accidentally, for example, upon phagosomal damage, whereas pathogens routinely accessing the host cytosol have evolved to avoid or even benefit from autophagy.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Autophagy in CD4+ T-cell immunity and tolerance.Cell Death Differ. 2009 Jan;16(1):79-86. doi: 10.1038/cdd.2008.113. Epub 2008 Jul 18. Cell Death Differ. 2009. PMID: 18636073 Review.
-
Manipulation of Autophagy by Bacterial Pathogens Impacts Host Immunity.Curr Issues Mol Biol. 2018;25:81-98. doi: 10.21775/cimb.025.081. Epub 2017 Sep 6. Curr Issues Mol Biol. 2018. PMID: 28875941 Review.
-
Autophagy in immunity and cell-autonomous defense against intracellular microbes.Immunol Rev. 2011 Mar;240(1):92-104. doi: 10.1111/j.1600-065X.2010.00995.x. Immunol Rev. 2011. PMID: 21349088 Free PMC article. Review.
-
Autophagy in innate and adaptive immunity against intracellular pathogens.J Mol Med (Berl). 2006 Mar;84(3):194-202. doi: 10.1007/s00109-005-0014-4. Epub 2006 Jan 28. J Mol Med (Berl). 2006. PMID: 16501849 Review.
-
Innate and adaptive immunity through autophagy.Immunity. 2007 Jul;27(1):11-21. doi: 10.1016/j.immuni.2007.07.004. Immunity. 2007. PMID: 17663981 Free PMC article. Review.
Cited by
-
Interactions between Autophagy and Bacterial Toxins: Targets for Therapy?Toxins (Basel). 2015 Aug 4;7(8):2918-58. doi: 10.3390/toxins7082918. Toxins (Basel). 2015. PMID: 26248079 Free PMC article. Review.
-
Combination therapies enhance immunoregulatory properties of MIAMI cells.Stem Cell Res Ther. 2019 Dec 18;10(1):395. doi: 10.1186/s13287-019-1515-3. Stem Cell Res Ther. 2019. PMID: 31852519 Free PMC article.
-
Autophagy in HCV infection: keeping fat and inflammation at bay.Biomed Res Int. 2014;2014:265353. doi: 10.1155/2014/265353. Epub 2014 Aug 5. Biomed Res Int. 2014. PMID: 25162004 Free PMC article. Review.
-
Selective autophagy receptor SQSTM1/ p62 inhibits Seneca Valley virus replication by targeting viral VP1 and VP3.Autophagy. 2021 Nov;17(11):3763-3775. doi: 10.1080/15548627.2021.1897223. Epub 2021 Mar 14. Autophagy. 2021. PMID: 33719859 Free PMC article.
-
Bromide impairs the circadian clock and glycolytic homeostasis via disruption of autophagy in rat H9C2 cardiomyocytes.BMC Mol Cell Biol. 2020 Jun 19;21(1):44. doi: 10.1186/s12860-020-00289-8. BMC Mol Cell Biol. 2020. PMID: 32560625 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

