Proteasome-dependent disruption of the E3 ubiquitin ligase anaphase-promoting complex by HCMV protein pUL21a
- PMID: 22792066
- PMCID: PMC3390409
- DOI: 10.1371/journal.ppat.1002789
Proteasome-dependent disruption of the E3 ubiquitin ligase anaphase-promoting complex by HCMV protein pUL21a
Abstract
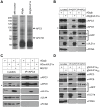
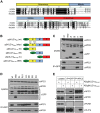
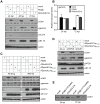
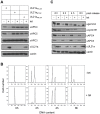
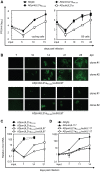
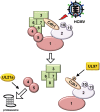
The anaphase-promoting complex (APC) is an E3 ubiquitin ligase which controls ubiquitination and degradation of multiple cell cycle regulatory proteins. During infection, human cytomegalovirus (HCMV), a widespread pathogen, not only phosphorylates the APC coactivator Cdh1 via the multifunctional viral kinase pUL97, it also promotes degradation of APC subunits via an unknown mechanism. Using a proteomics approach, we found that a recently identified HCMV protein, pUL21a, interacted with the APC. Importantly, we determined that expression of pUL21a was necessary and sufficient for proteasome-dependent degradation of APC subunits APC4 and APC5. This resulted in APC disruption and required pUL21a binding to the APC. We have identified the proline-arginine amino acid pair at residues 109-110 in pUL21a to be critical for its ability to bind and regulate the APC. A point mutant virus in which proline-arginine were mutated to alanines (PR-AA) grew at wild-type levels. However, a double mutant virus in which the viral ability to regulate the APC was abrogated by both PR-AA point mutation and UL97 deletion was markedly more attenuated compared to the UL97 deletion virus alone. This suggests that these mutations are synthetically lethal, and that HCMV exploits two viral factors to ensure successful disruption of the APC to overcome its restriction on virus infection. This study reveals the HCMV protein pUL21a as a novel APC regulator and uncovers a unique viral mechanism to subvert APC activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Studies on the Contribution of Human Cytomegalovirus UL21a and UL97 to Viral Growth and Inactivation of the Anaphase-Promoting Complex/Cyclosome (APC/C) E3 Ubiquitin Ligase Reveal a Unique Cellular Mechanism for Downmodulation of the APC/C Subunits APC1, APC4, and APC5.J Virol. 2015 Jul;89(13):6928-39. doi: 10.1128/JVI.00403-15. Epub 2015 Apr 22. J Virol. 2015. PMID: 25903336 Free PMC article.
-
Inactivation and disassembly of the anaphase-promoting complex during human cytomegalovirus infection is associated with degradation of the APC5 and APC4 subunits and does not require UL97-mediated phosphorylation of Cdh1.J Virol. 2010 Oct;84(20):10832-43. doi: 10.1128/JVI.01260-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686030 Free PMC article.
-
TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1.Curr Biol. 2003 Sep 2;13(17):1459-68. doi: 10.1016/s0960-9822(03)00581-5. Curr Biol. 2003. PMID: 12956947
-
Control the host cell cycle: viral regulation of the anaphase-promoting complex.J Virol. 2013 Aug;87(16):8818-25. doi: 10.1128/JVI.00088-13. Epub 2013 Jun 12. J Virol. 2013. PMID: 23760246 Free PMC article. Review.
-
Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis.Biochim Biophys Acta. 2014 Apr;1845(2):277-93. doi: 10.1016/j.bbcan.2014.02.001. Epub 2014 Feb 22. Biochim Biophys Acta. 2014. PMID: 24569229 Free PMC article. Review.
Cited by
-
Discovery of host-viral protein complexes during infection.Methods Mol Biol. 2013;1064:43-70. doi: 10.1007/978-1-62703-601-6_4. Methods Mol Biol. 2013. PMID: 23996249 Free PMC article.
-
Human cytomegalovirus IE1 protein disrupts interleukin-6 signaling by sequestering STAT3 in the nucleus.J Virol. 2013 Oct;87(19):10763-76. doi: 10.1128/JVI.01197-13. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903834 Free PMC article.
-
PUL21a-Cyclin A2 interaction is required to protect human cytomegalovirus-infected cells from the deleterious consequences of mitotic entry.PLoS Pathog. 2014 Nov 13;10(10):e1004514. doi: 10.1371/journal.ppat.1004514. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25393019 Free PMC article.
-
Synthetic lethal mutations in the cyclin A interface of human cytomegalovirus.PLoS Pathog. 2017 Jan 27;13(1):e1006193. doi: 10.1371/journal.ppat.1006193. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28129404 Free PMC article.
-
Human Cytomegalovirus Primary Infection and Reactivation: Insights From Virion-Carried Molecules.Front Microbiol. 2020 Jul 14;11:1511. doi: 10.3389/fmicb.2020.01511. eCollection 2020. Front Microbiol. 2020. PMID: 32765441 Free PMC article. Review.
References
-
- Blanchette P, Branton PE. Manipulation of the ubiquitin-proteasome pathway by small DNA tumor viruses. Virology. 2009;384:317–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

