Dysfunction of the ubiquitin ligase Ube3a may be associated with synaptic pathophysiology in a mouse model of Huntington disease
- PMID: 22787151
- PMCID: PMC3436189
- DOI: 10.1074/jbc.M112.371724
Dysfunction of the ubiquitin ligase Ube3a may be associated with synaptic pathophysiology in a mouse model of Huntington disease
Abstract
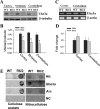
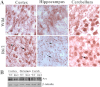
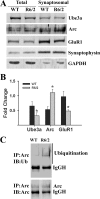
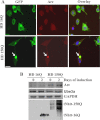
Huntington disease (HD) is a hereditary neurodegenerative disorder characterized by progressive cognitive, psychiatric, and motor symptoms. The disease is caused by abnormal expansion of CAG repeats in the gene encoding huntingtin, but how mutant huntingtin leads to early cognitive deficits in HD is poorly understood. Here, we demonstrate that the ubiquitin ligase Ube3a, which is implicated in synaptic plasticity and involved in the clearance of misfolded polyglutamine protein, is strongly recruited to the mutant huntingtin nuclear aggregates, resulting in significant loss of its functional pool in different regions of HD mouse brain. Interestingly, Arc, one of the substrates of Ube3a linked with synaptic plasticity, is also associated with nuclear aggregates, although its synaptic level is increased in the hippocampus and cortex of HD mouse brain. Different regions of HD mouse brain also exhibit decreased levels of AMPA receptors and various pre- and postsynaptic proteins, which could be due to the partial loss of function of Ube3a. Transient expression of mutant huntingtin in mouse primary cortical neurons further demonstrates recruitment of Ube3a into mutant huntingtin aggregates, increased accumulation of Arc, and decreased numbers of GluR1 puncta in the neuronal processes. Altogether, our results suggest that the loss of function of Ube3a might be associated with the synaptic abnormalities observed in HD.
Figures

Similar articles
-
Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington's disease.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1035-45. doi: 10.1098/rstb.1999.0456. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434303 Free PMC article.
-
Impaired ubiquitin-proteasome system activity in the synapses of Huntington's disease mice.J Cell Biol. 2008 Mar 24;180(6):1177-89. doi: 10.1083/jcb.200709080. J Cell Biol. 2008. PMID: 18362179 Free PMC article.
-
Early development of aberrant synaptic plasticity in a mouse model of Huntington's disease.Hum Mol Genet. 2006 May 15;15(10):1690-703. doi: 10.1093/hmg/ddl092. Epub 2006 Apr 6. Hum Mol Genet. 2006. PMID: 16600988
-
Dysregulation of synaptic proteins, dendritic spine abnormalities and pathological plasticity of synapses as experience-dependent mediators of cognitive and psychiatric symptoms in Huntington's disease.Neuroscience. 2013 Oct 22;251:66-74. doi: 10.1016/j.neuroscience.2012.05.043. Epub 2012 May 24. Neuroscience. 2013. PMID: 22633949 Review.
-
Synaptic dysfunction in Huntington's disease: a new perspective.Cell Mol Life Sci. 2005 Sep;62(17):1901-12. doi: 10.1007/s00018-005-5084-5. Cell Mol Life Sci. 2005. PMID: 15968465 Free PMC article. Review.
Cited by
-
LRSAM1 E3 ubiquitin ligase: molecular neurobiological perspectives linked with brain diseases.Cell Mol Life Sci. 2019 Jun;76(11):2093-2110. doi: 10.1007/s00018-019-03055-y. Epub 2019 Mar 2. Cell Mol Life Sci. 2019. PMID: 30826859 Free PMC article. Review.
-
Siah-1-interacting protein regulates mutated huntingtin protein aggregation in Huntington's disease models.Cell Biosci. 2022 Mar 19;12(1):34. doi: 10.1186/s13578-022-00755-0. Cell Biosci. 2022. PMID: 35305696 Free PMC article.
-
Azadiradione Restores Protein Quality Control and Ameliorates the Disease Pathogenesis in a Mouse Model of Huntington's Disease.Mol Neurobiol. 2018 Aug;55(8):6337-6346. doi: 10.1007/s12035-017-0853-3. Epub 2018 Jan 2. Mol Neurobiol. 2018. PMID: 29294248
-
Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A.Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5706-11. doi: 10.1073/pnas.1402215111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706802 Free PMC article.
-
Grb2 is regulated by foxd3 and has roles in preventing accumulation and aggregation of mutant huntingtin.PLoS One. 2013 Oct 8;8(10):e76792. doi: 10.1371/journal.pone.0076792. eCollection 2013. PLoS One. 2013. PMID: 24116161 Free PMC article.
References
-
- Huntington Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington disease chromosomes. Cell 72, 971–983 - PubMed
-
- Gatchel J. R., Zoghbi H. Y. (2005) Diseases of unstable repeat expansion: mechanisms and common principles. Nat. Rev. Genet. 6, 743–755 - PubMed
-
- Orr H. T., Zoghbi H. Y. (2007) Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621 - PubMed
-
- Cha J. H. (2000) Transcriptional dysregulation in Huntington disease. Trends Neurosci. 23, 387–392 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

