Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches
- PMID: 22778049
- PMCID: PMC4620732
- DOI: 10.1002/jor.22181
Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches
Abstract
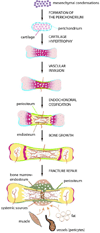
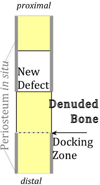
While century old clinical reports document the periosteum's remarkable regenerative capacity, only in the past decade have scientists undertaken mechanistic investigations of its regenerative potential. At a Workshop at the 2012 Annual Meeting of Orthopaedic Research Society, we reviewed the molecular, cellular, and tissue scale approaches to elucidate the mechanisms underlying the periosteum's regenerative potential as well as translational therapies engineering solutions inspired by its remarkable regenerative capacity. The entire population of osteoblasts within periosteum, and at endosteal and trabecular bone surfaces within the bone marrow, derives from the embryonic perichondrium. Periosteal cells contribute more to cartilage and bone formation within the callus during fracture healing than do cells of the bone marrow or endosteum, which do not migrate out of the marrow compartment. Furthermore, a current healing paradigm regards the activation, expansion, and differentiation of periosteal stem/progenitor cells as an essential step in building a template for subsequent neovascularization, bone formation, and remodeling. The periosteum comprises a complex, composite structure, providing a niche for pluripotent cells and a repository for molecular factors that modulate cell behavior. The periosteum's advanced, "smart" material properties change depending on the mechanical, chemical, and biological state of the tissue. Understanding periosteum development, progenitor cell-driven initiation of periosteum's endogenous tissue building capacity, and the complex structure-function relationships of periosteum as an advanced material are important for harnessing and engineering ersatz materials to mimic the periosteum's remarkable regenerative capacity.
Copyright © 2012 Orthopaedic Research Society.
Figures
Similar articles
-
Translating Periosteum's Regenerative Power: Insights From Quantitative Analysis of Tissue Genesis With a Periosteum Substitute Implant.Stem Cells Transl Med. 2016 Dec;5(12):1739-1749. doi: 10.5966/sctm.2016-0004. Epub 2016 Jul 27. Stem Cells Transl Med. 2016. PMID: 27465072 Free PMC article.
-
Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells.Stem Cells Transl Med. 2012 Jun;1(6):480-91. doi: 10.5966/sctm.2011-0056. Epub 2012 May 30. Stem Cells Transl Med. 2012. PMID: 23197852 Free PMC article. Review.
-
Elucidating multiscale periosteal mechanobiology: a key to unlocking the smart properties and regenerative capacity of the periosteum?Tissue Eng Part B Rev. 2013 Apr;19(2):147-59. doi: 10.1089/ten.TEB.2012.0216. Epub 2013 Feb 1. Tissue Eng Part B Rev. 2013. PMID: 23189933 Free PMC article. Review.
-
Live Tissue Imaging to Elucidate Mechanical Modulation of Stem Cell Niche Quiescence.Stem Cells Transl Med. 2017 Jan;6(1):285-292. doi: 10.5966/sctm.2015-0306. Epub 2016 Jul 28. Stem Cells Transl Med. 2017. PMID: 28170186 Free PMC article.
-
[Vascularized periosteum and bone regeneration].Chir Main. 2010 Dec;29 Suppl 1:S214-20. doi: 10.1016/j.main.2010.09.008. Epub 2010 Oct 8. Chir Main. 2010. PMID: 21075660 French.
Cited by
-
MMP9 regulates the cellular response to inflammation after skeletal injury.Bone. 2013 Jan;52(1):111-9. doi: 10.1016/j.bone.2012.09.018. Epub 2012 Sep 23. Bone. 2013. PMID: 23010105 Free PMC article.
-
Skeletal Blood Flow in Bone Repair and Maintenance.Bone Res. 2013 Dec 31;1(4):311-22. doi: 10.4248/BR201304002. eCollection 2013 Dec. Bone Res. 2013. PMID: 26273509 Free PMC article. Review.
-
The Fracture Callus Is Formed by Progenitors of Different Skeletal Origins in a Site-Specific Manner.JBMR Plus. 2019 May 4;3(9):e10193. doi: 10.1002/jbm4.10193. eCollection 2019 Sep. JBMR Plus. 2019. PMID: 31667451 Free PMC article.
-
Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin.Nat Commun. 2018 Feb 22;9(1):773. doi: 10.1038/s41467-018-03124-z. Nat Commun. 2018. PMID: 29472541 Free PMC article.
-
Periosteum mechanobiology and mechanistic insights for regenerative medicine.Bonekey Rep. 2016 Nov 30;5:857. doi: 10.1038/bonekey.2016.70. eCollection 2016. Bonekey Rep. 2016. PMID: 27974968 Free PMC article. Review.
References
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
-
- Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. - PubMed
-
- Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. - PubMed
-
- Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245–250. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

