Constitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood
- PMID: 22773837
- PMCID: PMC3436176
- DOI: 10.1074/jbc.M112.359125
Constitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood
Abstract
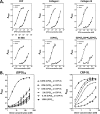
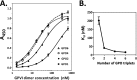
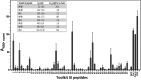
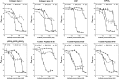
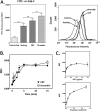
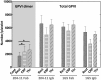
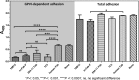
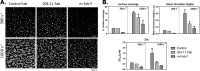
The platelet collagen receptor glycoprotein VI (GPVI) has been suggested to function as a dimer, with increased affinity for collagen. Dissociation constants (K(d)) obtained by measuring recombinant GPVI binding to collagenous substrates showed that GPVI dimers bind with high affinity to tandem GPO (Gly-Pro-Hyp) sequences in collagen, whereas the markedly lower affinity of the monomer for all substrates implies that it is not the collagen-binding form of GPVI. Dimer binding required a high density of immobilized triple-helical (GPO)(10)-containing peptide, suggesting that the dimer binds multiple, discrete peptide helices. Differential inhibition of dimer binding by dimer-specific antibodies, m-Fab-F and 204-11 Fab, suggests that m-Fab-F binds at the collagen-binding site of the dimer, and 204-11 Fab binds to a discrete site. Flow cytometric quantitation indicated that GPVI dimers account for ~29% of total GPVI in resting platelets, whereas activation by either collagen-related peptide or thrombin increases the number of dimers to ~39 and ~44%, respectively. m-Fab-F inhibits both GPVI-dependent static platelet adhesion to collagen and thrombus formation on collagen under low and high shear, indicating that pre-existing dimeric GPVI is required for the initial interaction with collagen because affinity of the monomer is too low to support binding and that interaction through the dimer is essential for platelet activation. These GPVI dimers in resting circulating platelets will enable them to bind injury-exposed subendothelial collagen to initiate platelet activation. The GPVI-specific agonist collagen-related peptide or thrombin further increases the number of dimers, thereby providing a feedback mechanism for reinforcing binding to collagen and platelet activation.
Figures


Similar articles
-
Clustering of glycoprotein VI (GPVI) dimers upon adhesion to collagen as a mechanism to regulate GPVI signaling in platelets.J Thromb Haemost. 2017 Mar;15(3):549-564. doi: 10.1111/jth.13613. Epub 2017 Feb 16. J Thromb Haemost. 2017. PMID: 28058806 Free PMC article.
-
Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation.J Thromb Haemost. 2018 Feb;16(2):389-404. doi: 10.1111/jth.13919. Epub 2018 Jan 15. J Thromb Haemost. 2018. PMID: 29210180 Free PMC article.
-
Glycoprotein (GP) VI dimer as a major collagen-binding site of native platelets: direct evidence obtained with dimeric GPVI-specific Fabs.J Thromb Haemost. 2009 Aug;7(8):1347-55. doi: 10.1111/j.1538-7836.2009.03496.x. Epub 2009 May 22. J Thromb Haemost. 2009. PMID: 19486274
-
Platelet glycoprotein VI.Adv Exp Med Biol. 2008;640:53-63. doi: 10.1007/978-0-387-09789-3_5. Adv Exp Med Biol. 2008. PMID: 19065783 Review.
-
Platelet glycoprotein VI: its structure and function.Thromb Res. 2004;114(4):221-33. doi: 10.1016/j.thromres.2004.06.046. Thromb Res. 2004. PMID: 15381385 Review.
Cited by
-
Rac Inhibition Causes Impaired GPVI Signalling in Human Platelets through GPVI Shedding and Reduction in PLCγ2 Phosphorylation.Int J Mol Sci. 2022 Mar 29;23(7):3746. doi: 10.3390/ijms23073746. Int J Mol Sci. 2022. PMID: 35409124 Free PMC article.
-
Activation-induced changes in platelet surface receptor expression and the contribution of the large-platelet subpopulation to activation.Res Pract Thromb Haemost. 2020 Jan 27;4(2):285-297. doi: 10.1002/rth2.12303. eCollection 2020 Feb. Res Pract Thromb Haemost. 2020. PMID: 32110760 Free PMC article.
-
Clustering of glycoprotein VI (GPVI) dimers upon adhesion to collagen as a mechanism to regulate GPVI signaling in platelets.J Thromb Haemost. 2017 Mar;15(3):549-564. doi: 10.1111/jth.13613. Epub 2017 Feb 16. J Thromb Haemost. 2017. PMID: 28058806 Free PMC article.
-
Two novel, putative mechanisms of action for citalopram-induced platelet inhibition.Sci Rep. 2018 Nov 12;8(1):16677. doi: 10.1038/s41598-018-34389-5. Sci Rep. 2018. PMID: 30420683 Free PMC article.
-
Platelet surface receptor glycoprotein VI-dimer is overexpressed in stroke: The Glycoprotein VI in Stroke (GYPSIE) study results.PLoS One. 2022 Jan 18;17(1):e0262695. doi: 10.1371/journal.pone.0262695. eCollection 2022. PLoS One. 2022. PMID: 35041713 Free PMC article.
References
-
- Sugiyama T., Okuma M., Ushikubi F., Sensaki S., Kanaji K., Uchino H. (1987) A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood 69, 1712–1720 - PubMed
-
- Watson S. P., Herbert J. M. J., Pollitt A. Y. (2010) GPVI and CLEC-2 in hemostasis and vascular integrity. J. Thromb. Haemost. 8, 1457–1467 - PubMed
-
- Clemetson J. M., Polgar J., Magnenat E., Wells T. N., Clemetson K. J. (1999) The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J. Biol. Chem. 274, 29019–29024 - PubMed
-
- Miura Y., Takahashi T., Jung S. M., Moroi M. (2002) Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 277, 46197–46204 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

