HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120
- PMID: 22773820
- PMCID: PMC3409750
- DOI: 10.1073/pnas.1204533109
HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120
Abstract
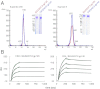
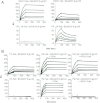
HIV-1 envelope glycoprotein is the primary target for HIV-1-specific antibodies. The native HIV-1 envelope spike on the virion surface is a trimer, but trimeric gp140 and monomeric gp120 currently are believed to induce comparable immune responses. Indeed, most studies on the immunogenicity of HIV-1 envelope oligomers have revealed only marginal improvement over monomers. We report here that suitably prepared envelope trimers have nearly all the antigenic properties expected for native viral spikes. These stable, rigorously homogenous trimers have antigenic properties markedly different from those of monomeric gp120s derived from the same sequences, and they induce potent neutralizing antibody responses for a cross-clade set of tier 1 and tier 2 viruses with titers substantially higher than those elicited by the corresponding gp120 monomers. These results, which demonstrate that there are relevant immunologic differences between monomers and high-quality envelope trimers, have important implications for HIV-1 vaccine development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Comparison of Uncleaved and Mature Human Immunodeficiency Virus Membrane Envelope Glycoprotein Trimers.J Virol. 2018 May 29;92(12):e00277-18. doi: 10.1128/JVI.00277-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618643 Free PMC article.
-
Immunogenicity of a Prefusion HIV-1 Envelope Trimer in Complex with a Quaternary-Structure-Specific Antibody.J Virol. 2015 Dec 30;90(6):2740-55. doi: 10.1128/JVI.02380-15. J Virol. 2015. PMID: 26719262 Free PMC article.
-
Selected HIV-1 Env trimeric formulations act as potent immunogens in a rabbit vaccination model.PLoS One. 2013 Sep 2;8(9):e74552. doi: 10.1371/journal.pone.0074552. eCollection 2013. PLoS One. 2013. PMID: 24023951 Free PMC article.
-
Conformational States of a Soluble, Uncleaved HIV-1 Envelope Trimer.J Virol. 2017 Apr 28;91(10):e00175-17. doi: 10.1128/JVI.00175-17. Print 2017 May 15. J Virol. 2017. PMID: 28250125 Free PMC article.
-
Immunogenicity of HIV-1 envelope glycoprotein oligomers.Curr Opin HIV AIDS. 2009 Sep;4(5):380-7. doi: 10.1097/COH.0b013e32832edc19. Curr Opin HIV AIDS. 2009. PMID: 20048701 Review.
Cited by
-
Natural infection as a blueprint for rational HIV vaccine design.Hum Vaccin Immunother. 2017 Jan 2;13(1):229-236. doi: 10.1080/21645515.2016.1232785. Epub 2016 Sep 20. Hum Vaccin Immunother. 2017. PMID: 27649455 Free PMC article. Review.
-
Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans.PLoS Pathog. 2013;9(5):e1003342. doi: 10.1371/journal.ppat.1003342. Epub 2013 May 2. PLoS Pathog. 2013. PMID: 23658524 Free PMC article.
-
Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1.J Virol. 2013 Apr;87(8):4185-201. doi: 10.1128/JVI.02297-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365441 Free PMC article.
-
Temsavir blocks the immunomodulatory activities of HIV-1 soluble gp120.Cell Chem Biol. 2023 May 18;30(5):540-552.e6. doi: 10.1016/j.chembiol.2023.03.003. Epub 2023 Mar 22. Cell Chem Biol. 2023. PMID: 36958337 Free PMC article.
-
Modulation of immune responses to liposomal vaccines by intrastructural help.Eur J Pharm Biopharm. 2023 Nov;192:112-125. doi: 10.1016/j.ejpb.2023.10.003. Epub 2023 Oct 4. Eur J Pharm Biopharm. 2023. PMID: 37797679 Free PMC article.
References
-
- Moore JP, McKeating JA, Weiss RA, Sattentau QJ. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. - PubMed
-
- Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

