Preferred WMSA catalytic mechanism of the nucleotidyl transfer reaction in human DNA polymerase κ elucidates error-free bypass of a bulky DNA lesion
- PMID: 22772988
- PMCID: PMC3467051
- DOI: 10.1093/nar/gks653
Preferred WMSA catalytic mechanism of the nucleotidyl transfer reaction in human DNA polymerase κ elucidates error-free bypass of a bulky DNA lesion
Abstract
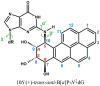
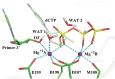
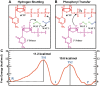
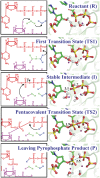
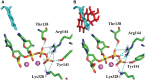
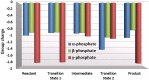
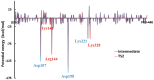
Human DNA Pol κ is a polymerase enzyme, specialized for near error-free bypass of certain bulky chemical lesions to DNA that are derived from environmental carcinogens present in tobacco smoke, automobile exhaust and cooked food. By employing ab initio QM/MM-MD (Quantum Mechanics/Molecular Mechanics-Molecular Dynamics) simulations with umbrella sampling, we have determined the entire free energy profile of the nucleotidyl transfer reaction catalyzed by Pol κ and provided detailed mechanistic insights. Our results show that a variant of the Water Mediated and Substrate Assisted (WMSA) mechanism that we previously deduced for Dpo4 and T7 DNA polymerases is preferred for Pol κ as well, suggesting its broad applicability. The hydrogen on the 3'-OH primer terminus is transferred through crystal and solvent waters to the γ-phosphate of the dNTP, followed by the associative nucleotidyl transfer reaction; this is facilitated by a proton transfer from the γ-phosphate to the α,β-bridging oxygen as pyrophosphate leaves, to neutralize the evolving negative charge. MD simulations show that the near error-free incorporation of dCTP opposite the major benzo[a]pyrene-derived dG lesion is compatible with the WMSA mechanism, allowing for an essentially undisturbed pentacovalent phosphorane transition state, and explaining the bypass of this lesion with little mutation by Pol κ.
Figures
Similar articles
-
The N-clasp of human DNA polymerase kappa promotes blockage or error-free bypass of adenine- or guanine-benzo[a]pyrenyl lesions.Nucleic Acids Res. 2008 Nov;36(20):6571-84. doi: 10.1093/nar/gkn719. Epub 2008 Oct 17. Nucleic Acids Res. 2008. PMID: 18931375 Free PMC article.
-
Polymerase-tailored variations in the water-mediated and substrate-assisted mechanism for nucleotidyl transfer: insights from a study of T7 DNA polymerase.J Mol Biol. 2009 Jun 19;389(4):787-96. doi: 10.1016/j.jmb.2009.04.029. Epub 2009 Apr 21. J Mol Biol. 2009. PMID: 19389406 Free PMC article.
-
Quantum mechanics/molecular mechanics investigation of the chemical reaction in Dpo4 reveals water-dependent pathways and requirements for active site reorganization.J Am Chem Soc. 2008 Oct 8;130(40):13240-50. doi: 10.1021/ja802215c. Epub 2008 Sep 12. J Am Chem Soc. 2008. PMID: 18785738 Free PMC article.
-
Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal.Chem Res Toxicol. 2009 May;22(5):759-78. doi: 10.1021/tx9000489. Chem Res Toxicol. 2009. PMID: 19397281 Free PMC article. Review.
-
Unlocking the sugar "steric gate" of DNA polymerases.Biochemistry. 2011 Feb 22;50(7):1135-42. doi: 10.1021/bi101915z. Epub 2011 Jan 26. Biochemistry. 2011. PMID: 21226515 Free PMC article. Review.
Cited by
-
Born-Oppenheimer Ab Initio QM/MM Molecular Dynamics Simulations of Enzyme Reactions.Methods Enzymol. 2016;577:105-18. doi: 10.1016/bs.mie.2016.05.013. Epub 2016 Jun 23. Methods Enzymol. 2016. PMID: 27498636 Free PMC article.
-
Computational Simulations of DNA Polymerases: Detailed Insights on Structure/Function/Mechanism from Native Proteins to Cancer Variants.Chem Res Toxicol. 2017 Nov 20;30(11):1922-1935. doi: 10.1021/acs.chemrestox.7b00161. Epub 2017 Sep 15. Chem Res Toxicol. 2017. PMID: 28877429 Free PMC article.
-
Variants of mouse DNA polymerase κ reveal a mechanism of efficient and accurate translesion synthesis past a benzo[a]pyrene dG adduct.Proc Natl Acad Sci U S A. 2014 Feb 4;111(5):1789-94. doi: 10.1073/pnas.1324168111. Epub 2014 Jan 21. Proc Natl Acad Sci U S A. 2014. PMID: 24449898 Free PMC article.
-
Applications of quantum mechanical/molecular mechanical methods to the chemical insertion step of DNA and RNA polymerization.Adv Protein Chem Struct Biol. 2014;97:83-113. doi: 10.1016/bs.apcsb.2014.10.001. Epub 2014 Nov 7. Adv Protein Chem Struct Biol. 2014. PMID: 25458356 Free PMC article. Review.
-
A new paradigm of DNA synthesis: three-metal-ion catalysis.Cell Biosci. 2016 Sep 6;6(1):51. doi: 10.1186/s13578-016-0118-2. eCollection 2016. Cell Biosci. 2016. PMID: 27602203 Free PMC article. Review.

