Results from pooled Phase III studies of ulipristal acetate for emergency contraception
- PMID: 22770793
- PMCID: PMC3766836
- DOI: 10.1016/j.contraception.2012.05.012
Results from pooled Phase III studies of ulipristal acetate for emergency contraception
Abstract
Background: Ulipristal acetate (UPA) is a new effective option to prevent unintended pregnancies up to 5 days after unprotected intercourse. We used pooled data from two Phase III studies to refine our understanding of the efficacy of UPA by time from unprotected intercourse and the effects of other factors on pregnancy rates.
Study design: Data from two Phase III studies were pooled to create a larger analysis population. Analyses were performed on the first participation of 2183 women.
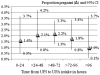
Results: A total of 41 women became pregnant despite the use of UPA, yielding an overall proportion pregnant of 1.9% (1.3%-2.5%). Proportions of pregnant women were higher among those with further acts of unprotected intercourse in the same cycle and among obese women. These varied from 1.3% (0.9%-2.0%) among nonobese women who had no further acts of unprotected intercourse (n=1704) to 8.3% (0.2%-38.5%) among obese women who had subsequent unprotected intercourse (n=12).
Conclusions: UPA is effective and safe in preventing pregnancy after unprotected intercourse. Its effectiveness is lower among women who have subsequent unprotected intercourse and among obese women.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception.Clin Ther. 2012 Jan;34(1):24-36. doi: 10.1016/j.clinthera.2011.11.012. Epub 2011 Dec 9. Clin Ther. 2012. PMID: 22154199 Review.
-
Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis.Lancet. 2010 Feb 13;375(9714):555-62. doi: 10.1016/S0140-6736(10)60101-8. Epub 2010 Jan 29. Lancet. 2010. PMID: 20116841 Clinical Trial.
-
[Emergency contraception: efficacy difference between levonorgestrel and ulipristal acetate depending on the follicular size at the time of an unprotected sexual intercourse].Gynecol Obstet Fertil. 2015 Mar;43(3):242-7. doi: 10.1016/j.gyobfe.2015.01.010. Epub 2015 Feb 18. Gynecol Obstet Fertil. 2015. PMID: 25703406 Review. French.
-
Canadian Contraception Consensus (Part 2 of 4).J Obstet Gynaecol Can. 2015 Nov;37(11):1033-9. doi: 10.1016/s1701-2163(16)30054-8. J Obstet Gynaecol Can. 2015. PMID: 26629725 English, French.
-
Canadian Contraception Consensus (Part 1 of 4).J Obstet Gynaecol Can. 2015 Oct;37(10):936-42. doi: 10.1016/s1701-2163(16)30033-0. J Obstet Gynaecol Can. 2015. PMID: 26606712 English, French.
Cited by
-
Safety and effectiveness data for emergency contraceptive pills among women with obesity: a systematic review.Contraception. 2016 Dec;94(6):605-611. doi: 10.1016/j.contraception.2016.05.002. Epub 2016 May 24. Contraception. 2016. PMID: 27234874 Free PMC article. Review.
-
Contraception and Reproductive Planning for Women With Cardiovascular Disease: JACC Focus Seminar 5/5.J Am Coll Cardiol. 2021 Apr 13;77(14):1823-1834. doi: 10.1016/j.jacc.2021.02.025. J Am Coll Cardiol. 2021. PMID: 33832608 Free PMC article. Review.
-
Interventions for emergency contraception.Cochrane Database Syst Rev. 2019 Jan 20;1(1):CD001324. doi: 10.1002/14651858.CD001324.pub6. Cochrane Database Syst Rev. 2019. PMID: 30661244 Free PMC article.
-
Hormonal contraceptives for contraception in overweight or obese women.Cochrane Database Syst Rev. 2016 Aug 18;2016(8):CD008452. doi: 10.1002/14651858.CD008452.pub4. Cochrane Database Syst Rev. 2016. PMID: 27537097 Free PMC article. Review.
-
Obesity and hormonal contraceptive efficacy.Womens Health (Lond). 2013 Sep;9(5):453-66. doi: 10.2217/whe.13.41. Womens Health (Lond). 2013. PMID: 24007251 Free PMC article. Review.
References
-
- Raymond E, Taylor D, Trussell J, Steiner MJ. Minimum effectiveness of the levonorgestrel regimen of emergency contraception. Contraception. 2004;69:79–81. - PubMed
-
- Piaggio G, Kapp N, von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: a combined analysis of four WHO trials. Contraception. 2011;84:35–9. - PubMed
-
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 http://dx.doi.org/10.1093/humrep/des140. - DOI - PMC - PubMed
-
- Fine P, Mathé H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115:257–63. - PubMed
-
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

