Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling
- PMID: 22763855
- PMCID: PMC3833451
- DOI: 10.1158/2159-8290.CD-12-0101
Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling
Abstract
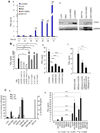
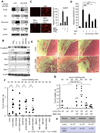
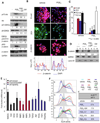
Mesenchymal cells of the tumor-associated stroma are critical determinants of carcinoma cell behavior. We focus here on interactions of carcinoma cells with mesenchymal stem cells (MSC), which are recruited to the tumor stroma and, once present, are able to influence the phenotype of the carcinoma cells. We find that carcinoma cell-derived interleukin-1 (IL-1) induces prostaglandin E(2) (PGE(2)) secretion by MSCs. The resulting PGE(2) operates in an autocrine manner, cooperating with ongoing paracrine IL-1 signaling, to induce expression of a group of cytokines by the MSCs. The PGE(2) and cytokines then proceed to act in a paracrine fashion on the carcinoma cells to induce activation of β-catenin signaling and formation of cancer stem cells. These observations indicate that MSCs and derived cell types create a cancer stem cell niche to enable tumor progression via release of PGE(2) and cytokines.
Significance: Although PGE2 has been implicated time and again in fostering tumorigenesis, its effects on carcinoma cells that contribute specifically to tumor formation are poorly understood. Here we show that tumor cells are able to elicit a strong induction of the COX-2/microsomal prostaglandin-E synthase-1 (mPGES-1)/PGE(2) axis in MSCs recruited to the tumor-associated stroma by releasing IL-1, which in turn elicits a mesenchymal/stem cell–like phenotype in the carcinoma cells.
Conflict of interest statement
Conflict of interest statement: No potential conflicts of interest were disclosed.
Figures




Comment in
-
Paracrine signaling between carcinoma cells and mesenchymal stem cells generates cancer stem cell niche via epithelial-mesenchymal transition.Cancer Discov. 2012 Sep;2(9):775-7. doi: 10.1158/2159-8290.CD-12-0312. Cancer Discov. 2012. PMID: 22969117 Free PMC article.
Similar articles
-
Paracrine signaling between carcinoma cells and mesenchymal stem cells generates cancer stem cell niche via epithelial-mesenchymal transition.Cancer Discov. 2012 Sep;2(9):775-7. doi: 10.1158/2159-8290.CD-12-0312. Cancer Discov. 2012. PMID: 22969117 Free PMC article.
-
Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche.Nature. 2020 Apr;580(7804):524-529. doi: 10.1038/s41586-020-2166-3. Epub 2020 Apr 1. Nature. 2020. PMID: 32322056 Free PMC article.
-
Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice.Gastroenterology. 2015 Dec;149(7):1884-1895.e4. doi: 10.1053/j.gastro.2015.07.064. Epub 2015 Aug 7. Gastroenterology. 2015. PMID: 26261008 Free PMC article.
-
Roles of cell fusion between mesenchymal stromal/stem cells and malignant cells in tumor growth and metastasis.FEBS J. 2021 Mar;288(5):1447-1456. doi: 10.1111/febs.15483. Epub 2020 Jul 24. FEBS J. 2021. PMID: 33070450 Review.
-
Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities.Biomed Res Int. 2019 May 8;2019:2820853. doi: 10.1155/2019/2820853. eCollection 2019. Biomed Res Int. 2019. PMID: 31205939 Free PMC article. Review.
Cited by
-
Targeting pancreatitis blocks tumor-initiating stem cells and pancreatic cancer progression.Oncotarget. 2015 Jun 20;6(17):15524-39. doi: 10.18632/oncotarget.3499. Oncotarget. 2015. PMID: 25906749 Free PMC article.
-
Dual Role of Fibroblasts Educated by Tumour in Cancer Behavior and Therapeutic Perspectives.Int J Mol Sci. 2022 Dec 8;23(24):15576. doi: 10.3390/ijms232415576. Int J Mol Sci. 2022. PMID: 36555218 Free PMC article. Review.
-
Cancer Stem Cells: An Ever-Hiding Foe.Exp Suppl. 2022;113:219-251. doi: 10.1007/978-3-030-91311-3_8. Exp Suppl. 2022. PMID: 35165866
-
The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment.Front Immunol. 2023 Feb 6;14:1074863. doi: 10.3389/fimmu.2023.1074863. eCollection 2023. Front Immunol. 2023. PMID: 36814921 Free PMC article. Review.
-
The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells.Biomedicines. 2023 Feb 3;11(2):445. doi: 10.3390/biomedicines11020445. Biomedicines. 2023. PMID: 36830980 Free PMC article. Review.
References
-
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

