Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons
- PMID: 22762025
- PMCID: PMC3385942
- DOI: 10.1101/cshperspect.a012070
Opiate-induced molecular and cellular plasticity of ventral tegmental area and locus coeruleus catecholamine neurons
Abstract
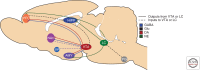
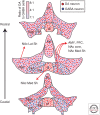
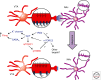
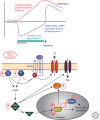
The study of neuronal adaptations induced by opiate drugs is particularly relevant today given their widespread prescription and nonprescription use. Although much is known about the acute actions of such drugs on the nervous system, a great deal of work remains to fully understand their chronic effects. Here, we focus on longer-lasting adaptations that occur in two catecholaminergic brain regions that mediate distinct behavioral actions of opiates: ventral tegmental area (VTA) dopaminergic neurons, important for drug reward, and locus coeruleus (LC) noradrenergic neurons, important for physical dependence and withdrawal. We focus on changes in cellular, synaptic, and structural plasticity in these brain regions that contribute to opiate dependence and addiction. Understanding the molecular determinants of this opiate-induced plasticity will be critical for the development of better treatments for opiate addiction and perhaps safer opiate drugs for medicinal use.
Figures
Similar articles
-
Opiates and Plasticity in the Ventral Tegmental Area.ACS Chem Neurosci. 2017 Sep 20;8(9):1830-1838. doi: 10.1021/acschemneuro.7b00281. Epub 2017 Aug 16. ACS Chem Neurosci. 2017. PMID: 28768409 Free PMC article. Review.
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
-
Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons.J Neurosci. 2002 May 15;22(10):4153-62. doi: 10.1523/JNEUROSCI.22-10-04153.2002. J Neurosci. 2002. PMID: 12019333 Free PMC article.
-
Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area.J Neurosci. 2005 Jun 22;25(25):6005-15. doi: 10.1523/JNEUROSCI.0062-05.2005. J Neurosci. 2005. PMID: 15976090 Free PMC article.
-
Opiates and plasticity.Neuropharmacology. 2011 Dec;61(7):1088-96. doi: 10.1016/j.neuropharm.2011.01.028. Epub 2011 Jan 25. Neuropharmacology. 2011. PMID: 21272593 Review.
Cited by
-
Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics.J Neurosci. 2015 Oct 14;35(41):13879-88. doi: 10.1523/JNEUROSCI.2711-15.2015. J Neurosci. 2015. PMID: 26468188 Free PMC article. Review.
-
Regulation of neurological and neuropsychiatric phenotypes by locus coeruleus-derived galanin.Brain Res. 2016 Jun 15;1641(Pt B):320-37. doi: 10.1016/j.brainres.2015.11.025. Epub 2015 Nov 20. Brain Res. 2016. PMID: 26607256 Free PMC article. Review.
-
Expression of the μ, κ, and δ-opioid receptors and tyrosine hydroxylase in MN9D cells.Int J Clin Exp Pathol. 2015 May 1;8(5):4863-8. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191179 Free PMC article.
-
Electrocardiographic Abnormalities During and After Withdrawal in Patients Diagnosed with Opioid Use Disorder.Noro Psikiyatr Ars. 2023 Mar 14;60(4):304-309. doi: 10.29399/npa.28365. eCollection 2023. Noro Psikiyatr Ars. 2023. PMID: 38077834 Free PMC article.
-
Characterisation of the Novel Mixed Mu-NOP Peptide Ligand Dermorphin-N/OFQ (DeNo).PLoS One. 2016 Jun 7;11(6):e0156897. doi: 10.1371/journal.pone.0156897. eCollection 2016. PLoS One. 2016. PMID: 27272042 Free PMC article.
References
-
- Aghajanian GK 1978. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature 276: 186–188 - PubMed
-
- Aghajanian GK, Kogan JH, Moghaddam B 1994. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: An in vivo microdialysis study. Brain Res 636: 126–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
