Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys
- PMID: 22761379
- PMCID: PMC3446565
- DOI: 10.1128/JVI.00996-12
Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys
Abstract
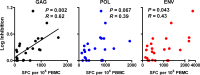
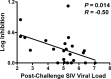
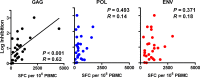
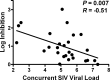
A comprehensive vaccine for human immunodeficiency virus type 1 (HIV-1) would block HIV-1 acquisition as well as durably control viral replication in breakthrough infections. Recent studies have demonstrated that Env is required for a vaccine to protect against acquisition of simian immunodeficiency virus (SIV) in vaccinated rhesus monkeys, but the antigen requirements for virologic control remain unclear. Here, we investigate whether CD8(+) T lymphocytes from vaccinated rhesus monkeys mediate viral inhibition in vitro and whether these responses predict virologic control following SIV challenge. We observed that CD8(+) lymphocytes from 23 vaccinated rhesus monkeys inhibited replication of SIV in vitro. Moreover, the magnitude of inhibition prior to challenge was inversely correlated with set point SIV plasma viral loads after challenge. In addition, CD8 cell-mediated viral inhibition in vaccinated rhesus monkeys correlated significantly with Gag-specific, but not Pol- or Env-specific, CD4(+) and CD8(+) T lymphocyte responses. These findings demonstrate that in vitro viral inhibition following vaccination largely reflects Gag-specific cellular immune responses and correlates with in vivo virologic control following infection. These data suggest the importance of including Gag in an HIV-1 vaccine in which virologic control is desired.
Figures


Similar articles
-
Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection.J Virol. 2000 Aug;74(16):7485-95. doi: 10.1128/jvi.74.16.7485-7495.2000. J Virol. 2000. PMID: 10906202 Free PMC article.
-
Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus.J Virol. 2011 Dec;85(23):12708-20. doi: 10.1128/JVI.00865-11. Epub 2011 Sep 7. J Virol. 2011. PMID: 21900170 Free PMC article.
-
Vaccination of Macaques with DNA Followed by Adenoviral Vectors Encoding Simian Immunodeficiency Virus (SIV) Gag Alone Delays Infection by Repeated Mucosal Challenge with SIV.J Virol. 2019 Oct 15;93(21):e00606-19. doi: 10.1128/JVI.00606-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413132 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Impact of MHC class I diversity on immune control of immunodeficiency virus replication.Nat Rev Immunol. 2008 Aug;8(8):619-30. doi: 10.1038/nri2357. Nat Rev Immunol. 2008. PMID: 18617886 Free PMC article. Review.
Cited by
-
Combination strategies to durably suppress HIV-1: Soluble T cell receptors.J Virus Erad. 2022 Aug 24;8(3):100082. doi: 10.1016/j.jve.2022.100082. eCollection 2022 Sep. J Virus Erad. 2022. PMID: 36065296 Free PMC article.
-
Virus-like vaccines against HIV/SIV synergize with a subdominant antigen T cell vaccine.J Transl Med. 2019 May 24;17(1):175. doi: 10.1186/s12967-019-1924-1. J Transl Med. 2019. PMID: 31126293 Free PMC article.
-
A cure for AIDS: a matter of timing?Retrovirology. 2013 Nov 22;10:145. doi: 10.1186/1742-4690-10-145. Retrovirology. 2013. PMID: 24267982 Free PMC article. Review.
-
Effect of vaginal immunization with HIVgp140 and HSP70 on HIV-1 replication and innate and T cell adaptive immunity in women.J Virol. 2014 Oct;88(20):11648-57. doi: 10.1128/JVI.01621-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008917 Free PMC article.
-
Development and Preclinical Evaluation of an Integrase Defective Lentiviral Vector Vaccine Expressing the HIVACAT T Cell Immunogen in Mice.Mol Ther Methods Clin Dev. 2020 Feb 4;17:418-428. doi: 10.1016/j.omtm.2020.01.013. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32154327 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- U19 AI078526/AI/NIAID NIH HHS/United States
- AI095985/AI/NIAID NIH HHS/United States
- AI066305/AI/NIAID NIH HHS/United States
- R01 AI066924/AI/NIAID NIH HHS/United States
- U19 AI095985/AI/NIAID NIH HHS/United States
- AI030914/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI030914/AI/NIAID NIH HHS/United States
- U19 AI066305/AI/NIAID NIH HHS/United States
- AI066924/AI/NIAID NIH HHS/United States
- AI07387/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

