The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host
- PMID: 22754550
- PMCID: PMC3384971
- DOI: 10.3389/fmicb.2012.00223
The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host
Abstract
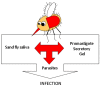
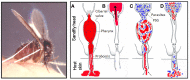
Leishmania are transmitted by the bite of their sand fly vector and this has a significant influence on the virulence of the resulting infection. From our studies into the interaction between parasite, vector, and host we have uncovered an important missing ingredient during Leishmania transmission. Leishmania actively adapt their sand fly hosts into efficient vectors by secreting Promastigote Secretory Gel (PSG), a proteophosphoglycan (PPG)-rich, mucin-like gel which accumulates in sand fly gut and mouthparts. This has the effect of blocking the fly, such that during bloodfeeding both parasites and gel are co-transmitted in an act of regurgitation. We are discovering that this has further implications for the mammalian infection, again, in favor of the parasite. Experimentally, PSG exacerbates cutaneous and visceral leishmaniasis and can promote the chronicity of Leishmania infection, even in mouse strains normally capable of controlling leishmaniasis. The underlying mechanism of PSG's action is a major focus of our ongoing work. This review aims to synthesize what is known about the role and action of PSG and its constituent proteophosphoglycans, for parasite colonization of the sand fly, transmission, and mammalian infection. Lastly, we discuss potential exploitation of this important vector-transmitted product and future avenues of research.
Keywords: Leishmania; macrophage; promastigote secretory gel; proteophosphoglycan; saliva; sand fly; transmission; wound healing.
Figures


Similar articles
-
Promastigote secretory gel from natural and unnatural sand fly vectors exacerbate Leishmania major and Leishmania tropica cutaneous leishmaniasis in mice.Parasitology. 2019 Dec;146(14):1796-1802. doi: 10.1017/S0031182019001069. Epub 2019 Aug 29. Parasitology. 2019. PMID: 31452467 Free PMC article.
-
Leishmania proteophosphoglycans regurgitated from infected sand flies accelerate dermal wound repair and exacerbate leishmaniasis via insulin-like growth factor 1-dependent signalling.PLoS Pathog. 2018 Jan 19;14(1):e1006794. doi: 10.1371/journal.ppat.1006794. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29352310 Free PMC article.
-
Proteophosophoglycans regurgitated by Leishmania-infected sand flies target the L-arginine metabolism of host macrophages to promote parasite survival.PLoS Pathog. 2009 Aug;5(8):e1000555. doi: 10.1371/journal.ppat.1000555. Epub 2009 Aug 21. PLoS Pathog. 2009. PMID: 19696894 Free PMC article.
-
Gels and cells: the Leishmania biofilm as a space and place for parasite transmission.Trends Parasitol. 2024 Oct;40(10):876-885. doi: 10.1016/j.pt.2024.08.001. Epub 2024 Aug 31. Trends Parasitol. 2024. PMID: 39218719 Review.
-
Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform leishmaniasis transmission dynamics and control.PLoS Pathog. 2017 Oct 19;13(10):e1006571. doi: 10.1371/journal.ppat.1006571. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29049371 Free PMC article. Review.
Cited by
-
Leishmania kinetoplast DNA contributes to parasite burden in infected macrophages: Critical role of the cGAS-STING-TBK1 signaling pathway in macrophage parasitemia.Front Immunol. 2022 Nov 2;13:1007070. doi: 10.3389/fimmu.2022.1007070. eCollection 2022. Front Immunol. 2022. PMID: 36405710 Free PMC article.
-
Promastigote secretory gel from natural and unnatural sand fly vectors exacerbate Leishmania major and Leishmania tropica cutaneous leishmaniasis in mice.Parasitology. 2019 Dec;146(14):1796-1802. doi: 10.1017/S0031182019001069. Epub 2019 Aug 29. Parasitology. 2019. PMID: 31452467 Free PMC article.
-
Differential Regulation of l-Arginine Metabolism through Arginase 1 during Infection with Leishmania mexicana Isolates Obtained from Patients with Localized and Diffuse Cutaneous Leishmaniasis.Infect Immun. 2020 Jun 22;88(7):e00963-19. doi: 10.1128/IAI.00963-19. Print 2020 Jun 22. Infect Immun. 2020. PMID: 32312763 Free PMC article.
-
Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity.Nat Microbiol. 2018 May;3(5):548-555. doi: 10.1038/s41564-018-0125-7. Epub 2018 Mar 19. Nat Microbiol. 2018. PMID: 29556108 Free PMC article.
-
The role of dermis resident macrophages and their interaction with neutrophils in the early establishment of Leishmania major infection transmitted by sand fly bite.PLoS Pathog. 2020 Nov 2;16(11):e1008674. doi: 10.1371/journal.ppat.1008674. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33137149 Free PMC article.
References
-
- Anderson R. A., Knols B. G., Koella J. C. (2000). Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology 120, 329–333 - PubMed
-
- Ashford R. W., Kohestany K. A., Karimzad M. A. (1992). Cutaneous leishmaniasis in Kabul: observations on a “prolonged epidemic”. Ann. Trop. Med. Parasitol. 86, 361–371 - PubMed
-
- Barral A., Carvalho E. M., Badaro R., Barral-Netto M. (1986). Suppression of lymphocyte proliferative responses by sera from patients with American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 35, 735–742 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

