Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones
- PMID: 22752453
- PMCID: PMC3441952
- DOI: 10.1007/s10162-012-0337-0
Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones
Abstract
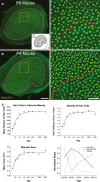
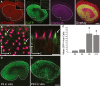
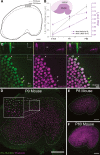
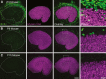
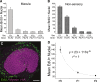
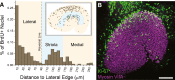
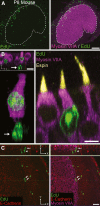
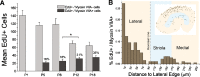
Many non-mammalian vertebrates produce hair cells throughout life and recover from hearing and balance deficits through regeneration. In contrast, embryonic production of hair cells declines sharply in mammals where deficits from hair cell losses are typically permanent. Hair cell density estimates recently suggested that the vestibular organs of mice continue to add hair cells after birth, so we undertook comprehensive counting in murine utricles at different ages. The counts show that 51% of the hair cells in adults arise during the 2 weeks after birth. Immature hair cells are most common near the neonatal macula's peripheral edge and striola, where anti-Ki-67 labels cycling nuclei in zones that appear to contain niches for supporting-cell-like stem cells. In vivo lineage tracing in a novel reporter mouse where tamoxifen-inducible supporting cell-specific Cre expression switched tdTomato fluorescence to eGFP fluorescence showed that proteolipid-protein-1-expressing supporting cells are an important source of the new hair cells. To assess the contributions of postnatal cell divisions, we gave mice an injection of BrdU or EdU on the day of birth. The labels were restricted to supporting cells 1 day later, but by 12 days, 31% of the labeled nuclei were in myosin-VIIA-positive hair cells. Thus, hair cell populations in neonatal mouse utricles grow appreciably through two processes: the progressive differentiation of cells generated before birth and the differentiation of new cells arising from divisions of progenitors that progress through S phase soon after birth. Subsequent declines in these processes coincide with maturational changes that appear unique to mammalian supporting cells.
Figures
Similar articles
-
In vivo proliferative regeneration of balance hair cells in newborn mice.J Neurosci. 2012 May 9;32(19):6570-7. doi: 10.1523/JNEUROSCI.6274-11.2012. J Neurosci. 2012. PMID: 22573679 Free PMC article.
-
YAP Mediates Hair Cell Regeneration in Balance Organs of Chickens, But LATS Kinases Suppress Its Activity in Mice.J Neurosci. 2020 May 13;40(20):3915-3932. doi: 10.1523/JNEUROSCI.0306-20.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341094 Free PMC article.
-
Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity.Hear Res. 2009 Jan;247(1):17-26. doi: 10.1016/j.heares.2008.08.010. Epub 2008 Sep 7. Hear Res. 2009. PMID: 18809482 Free PMC article.
-
Development and regeneration of the inner ear.Ann N Y Acad Sci. 2009 Jul;1170:28-33. doi: 10.1111/j.1749-6632.2009.04484.x. Ann N Y Acad Sci. 2009. PMID: 19686102 Free PMC article. Review.
-
Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species.Hear Res. 2011 Mar;273(1-2):72-9. doi: 10.1016/j.heares.2010.05.004. Epub 2010 May 19. Hear Res. 2011. PMID: 20488231 Review.
Cited by
-
Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice.Elife. 2017 Mar 6;6:e18128. doi: 10.7554/eLife.18128. Elife. 2017. PMID: 28263708 Free PMC article.
-
A historical to present-day account of efforts to answer the question: "what puts the brakes on mammalian hair cell regeneration?".Hear Res. 2013 Mar;297:52-67. doi: 10.1016/j.heares.2013.01.005. Epub 2013 Jan 17. Hear Res. 2013. PMID: 23333259 Free PMC article. Review.
-
PCP Auto Count: A Novel Fiji/ImageJ plug-in for automated quantification of planar cell polarity and cell counting.bioRxiv [Preprint]. 2024 Feb 15:2024.01.30.578047. doi: 10.1101/2024.01.30.578047. bioRxiv. 2024. Update in: Front Cell Dev Biol. 2024 May 17;12:1394031. doi: 10.3389/fcell.2024.1394031 PMID: 38352473 Free PMC article. Updated. Preprint.
-
Transdifferentiation is uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear.bioRxiv [Preprint]. 2024 Apr 11:2024.04.09.588777. doi: 10.1101/2024.04.09.588777. bioRxiv. 2024. Update in: Development. 2024 Aug 1;151(15):dev202944. doi: 10.1242/dev.202944 PMID: 38645220 Free PMC article. Updated. Preprint.
-
Rescue of Outer Hair Cells with Antisense Oligonucleotides in Usher Mice Is Dependent on Age of Treatment.J Assoc Res Otolaryngol. 2018 Feb;19(1):1-16. doi: 10.1007/s10162-017-0640-x. Epub 2017 Oct 12. J Assoc Res Otolaryngol. 2018. PMID: 29027038 Free PMC article.
References
-
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

