Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis
- PMID: 22748923
- PMCID: PMC3526191
- DOI: 10.1016/j.molcel.2012.05.041
Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis
Abstract
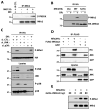
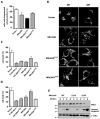
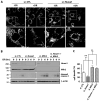
Mitochondria play central roles in integrating pro- and antiapoptotic stimuli, and JNK is well known to have roles in activating apoptotic pathways. We establish a critical link between stress-induced JNK activation, mitofusin 2, which is an essential component of the mitochondrial outer membrane fusion apparatus, and the ubiquitin-proteasome system (UPS). JNK phosphorylation of mitofusin 2 in response to cellular stress leads to recruitment of the ubiquitin ligase (E3) Huwe1/Mule/ARF-BP1/HectH9/E3Histone/Lasu1 to mitofusin 2, with the BH3 domain of Huwe1 implicated in this interaction. This results in ubiquitin-mediated proteasomal degradation of mitofusin 2, leading to mitochondrial fragmentation and enhanced apoptotic cell death. The stability of a nonphosphorylatable mitofusin 2 mutant is unaffected by stress and protective against apoptosis. Conversely, a mitofusin 2 phosphomimic is more rapidly degraded without cellular stress. These findings demonstrate how proximal signaling events can influence both mitochondrial dynamics and apoptosis through phosphorylation-stimulated degradation of the mitochondrial fusion machinery.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article.
-
Rapid degradation of mutant SLC25A46 by the ubiquitin-proteasome system results in MFN1/2-mediated hyperfusion of mitochondria.Mol Biol Cell. 2017 Mar 1;28(5):600-612. doi: 10.1091/mbc.E16-07-0545. Epub 2017 Jan 5. Mol Biol Cell. 2017. PMID: 28057766 Free PMC article.
-
Mitochondrial E3 ubiquitin ligase MARCH5 controls mitochondrial fission and cell sensitivity to stress-induced apoptosis through regulation of MiD49 protein.Mol Biol Cell. 2016 Jan 15;27(2):349-59. doi: 10.1091/mbc.E15-09-0678. Epub 2015 Nov 12. Mol Biol Cell. 2016. PMID: 26564796 Free PMC article.
-
Mitochondrial Ubiquitin Ligase in Cardiovascular Disorders.Adv Exp Med Biol. 2017;982:327-333. doi: 10.1007/978-3-319-55330-6_17. Adv Exp Med Biol. 2017. PMID: 28551795 Review.
-
Mitochondrial quality control by the ubiquitin-proteasome system.Biochem Soc Trans. 2011 Oct;39(5):1509-13. doi: 10.1042/BST0391509. Biochem Soc Trans. 2011. PMID: 21936843 Review.
Cited by
-
SUMOylation of MFF coordinates fission complexes to promote stress-induced mitochondrial fragmentation.Sci Adv. 2024 Oct 4;10(40):eadq6223. doi: 10.1126/sciadv.adq6223. Epub 2024 Oct 4. Sci Adv. 2024. PMID: 39365854 Free PMC article.
-
The role of ultrasound and mitofusin-2 levels to predict pregnancy outcomes in patients with severe preeclampsia: a case-control study.Rev Assoc Med Bras (1992). 2024 Aug 16;70(8):e20240152. doi: 10.1590/1806-9282.20240152. eCollection 2024. Rev Assoc Med Bras (1992). 2024. PMID: 39166673 Free PMC article.
-
Mitochondrial quality control in human health and disease.Mil Med Res. 2024 May 29;11(1):32. doi: 10.1186/s40779-024-00536-5. Mil Med Res. 2024. PMID: 38812059 Free PMC article. Review.
-
Kinase signalling adaptation supports dysfunctional mitochondria in disease.Front Mol Biosci. 2024 Jan 26;11:1354682. doi: 10.3389/fmolb.2024.1354682. eCollection 2024. Front Mol Biosci. 2024. PMID: 38434478 Free PMC article. Review.
-
Altered vacuole membrane protein 1 (VMP1) expression is associated with increased NLRP3 inflammasome activation and mitochondrial dysfunction.Inflamm Res. 2024 Apr;73(4):563-580. doi: 10.1007/s00011-024-01856-x. Epub 2024 Feb 27. Inflamm Res. 2024. PMID: 38411635
References
-
- Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, Kogel U, Scheffner M, Helin K, Eilers M. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell. 2005;123:409–421. - PubMed
-
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. - PubMed
-
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. - PubMed
-
- Casasnovas C, Banchs I, Cassereau J, Gueguen N, Chevrollier A, Martinez-Matos JA, Bonneau D, Volpini V. Phenotypic Spectrum of MFN2 Mutations in the Spanish Population. J Med Genet 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

