RNAi in the regulation of mammalian viral infections
- PMID: 22734679
- PMCID: PMC3383472
- DOI: 10.1186/1741-7007-10-58
RNAi in the regulation of mammalian viral infections
Abstract
Although RNA interference (RNAi) is known to play an important part in defense against viruses of invertebrates, its contribution to mammalian anti-viral defense has been a matter of dispute. This is surprising because all components of the RNAi machinery necessary for robust RNAi-mediated restriction of viruses are conserved in mammals, and the introduction of synthetic small interfering RNAs (siRNAs) into cells efficiently silences the replication of viruses that contain siRNA complementary sequences in those cells. Here, I discuss the reasons for the dispute, and review the evidence that RNAi is a part of the physiological defense of mammalian cells against viral infections.
Figures
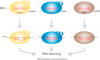
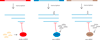
Similar articles
-
The silent treatment: RNAi as a defense against virus infection in mammals.Trends Biotechnol. 2006 Apr;24(4):186-93. doi: 10.1016/j.tibtech.2006.02.006. Epub 2006 Feb 28. Trends Biotechnol. 2006. PMID: 16503061 Review.
-
Antiviral innate immune response of RNA interference.J Infect Dev Ctries. 2014 Jul 14;8(7):804-10. doi: 10.3855/jidc.4187. J Infect Dev Ctries. 2014. PMID: 25022288 Review.
-
Slicing and dicing viruses: antiviral RNA interference in mammals.EMBO J. 2019 Apr 15;38(8):e100941. doi: 10.15252/embj.2018100941. Epub 2019 Mar 14. EMBO J. 2019. PMID: 30872283 Free PMC article. Review.
-
RNA-based mechanisms regulating host-virus interactions.Immunol Rev. 2013 May;253(1):97-111. doi: 10.1111/imr.12053. Immunol Rev. 2013. PMID: 23550641 Free PMC article. Review.
-
RNA-based viral immunity initiated by the Dicer family of host immune receptors.Immunol Rev. 2009 Jan;227(1):176-88. doi: 10.1111/j.1600-065X.2008.00722.x. Immunol Rev. 2009. PMID: 19120484 Free PMC article. Review.
Cited by
-
MicroRNAs and HIV-1: complex interactions.J Biol Chem. 2012 Nov 30;287(49):40884-90. doi: 10.1074/jbc.R112.415448. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043098 Free PMC article. Review.
-
Cleavage of Dicer protein by I7 protease during vaccinia virus infection.PLoS One. 2015 Mar 27;10(3):e0120390. doi: 10.1371/journal.pone.0120390. eCollection 2015. PLoS One. 2015. PMID: 25815818 Free PMC article.
-
RNAi: antiviral therapy against dengue virus.Asian Pac J Trop Biomed. 2013 Mar;3(3):232-6. doi: 10.1016/S2221-1691(13)60057-X. Asian Pac J Trop Biomed. 2013. PMID: 23620845 Free PMC article. Review.
-
Cellular RNA helicases and HIV-1: insights from genome-wide, proteomic, and molecular studies.Virus Res. 2013 Feb;171(2):357-65. doi: 10.1016/j.virusres.2012.06.022. Epub 2012 Jul 16. Virus Res. 2013. PMID: 22814432 Free PMC article. Review.
-
Small interfering RNA (siRNA)-based therapeutic applications against viruses: principles, potential, and challenges.J Biomed Sci. 2023 Oct 16;30(1):88. doi: 10.1186/s12929-023-00981-9. J Biomed Sci. 2023. PMID: 37845731 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

