FTY720 markedly increases alloengraftment but does not eliminate host anti-donor T cells that cause graft rejection on its withdrawal
- PMID: 22728248
- PMCID: PMC3520609
- DOI: 10.1016/j.bbmt.2012.06.007
FTY720 markedly increases alloengraftment but does not eliminate host anti-donor T cells that cause graft rejection on its withdrawal
Abstract
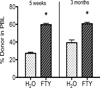
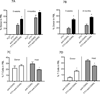
The immunomodulator FTY720 (FTY) is beneficial in models of graft-versus-host disease, solid organ transplantation, and autoimmunity and has been approved for use in patients with multiple sclerosis. FTY modifies the homing and migration of many cell types. We report that FTY has profound positive and negative effects on allogeneic bone marrow (BM) engraftment in sublethally irradiated recipients. FTY increased donor hematopoietic progenitors in the BM, resulting in high donor engraftment in the B cell, myeloid cell, and natural killer cell, but not T cell, lineages. Donor T cell progenitors within the thymus of FTY-treated recipients were dramatically reduced, resulting in a lack of donor T cell reconstitution. In addition to preventing the ingress of donor (and host) T cell progenitors, FTY prevented the egress of fully functional host CD4+CD8- and CD4-CD8+ thymocytes that on cessation of FTY administration were able to exit from the thymus and contribute to a rapid and complete rejection of a well-established donor BM graft. When used in combination with anti-CD40L mAbs to block the CD40L:CD40 costimulatory pathway, FTY markedly enhanced anti-CD40L mAb-mediated alloengraftment promotion. In contrast to FTY alone, the combination of anti-CD40L mAb and FTY resulted in a surprisingly stable, multilineage, long-term donor chimerism. These data illustrate FTY's profound migration modulating effects and suggest a use in combinatorial therapy in achieving stable alloengraftment under nonmyeloablative conditions.
Copyright © 2012 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of Conflict of Interest:
The authors have nothing to disclose.
Figures






Similar articles
-
Combined effects of calcineurin inhibitors or sirolimus with anti-CD40L mAb on alloengraftment under nonmyeloablative conditions.Blood. 2002 Nov 1;100(9):3400-7. doi: 10.1182/blood-2002-03-0872. Blood. 2002. PMID: 12384443
-
Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD).Blood. 2007 Nov 1;110(9):3480-8. doi: 10.1182/blood-2007-05-087940. Epub 2007 Jul 2. Blood. 2007. PMID: 17606761 Free PMC article.
-
Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions.Blood. 2001 Jul 15;98(2):467-74. doi: 10.1182/blood.v98.2.467. Blood. 2001. PMID: 11435318
-
Hematopoietic cell transplantation for the induction of allo- and xenotolerance.Clin Transplant. 1996 Aug;10(4):357-63. Clin Transplant. 1996. PMID: 8884109 Review.
-
Achieving alloengraftment without graft-versus-host disease: approaches using mixed allogeneic bone marrow transplantation.Bone Marrow Transplant. 1988 Sep;3(5):379-86. Bone Marrow Transplant. 1988. PMID: 3056545 Review.
Cited by
-
Immunomodulatory Effects of Bendamustine in Hematopoietic Cell Transplantation.Cancers (Basel). 2021 Apr 3;13(7):1702. doi: 10.3390/cancers13071702. Cancers (Basel). 2021. PMID: 33916711 Free PMC article. Review.
-
Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation.Int J Mol Sci. 2017 Sep 21;18(10):2027. doi: 10.3390/ijms18102027. Int J Mol Sci. 2017. PMID: 28934113 Free PMC article. Review.
-
Translational Implications for Off-the-shelf Immune Cells Expressing Chimeric Antigen Receptors.Mol Ther. 2016 Aug;24(7):1178-86. doi: 10.1038/mt.2016.106. Epub 2016 May 16. Mol Ther. 2016. PMID: 27203439 Free PMC article. Review.
-
Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy.Immunol Rev. 2017 Mar;276(1):192-212. doi: 10.1111/imr.12523. Immunol Rev. 2017. PMID: 28258702 Free PMC article. Review.
-
Preclinical models of acute and chronic graft-versus-host disease: how predictive are they for a successful clinical translation?Blood. 2016 Jun 23;127(25):3117-26. doi: 10.1182/blood-2016-02-699082. Epub 2016 Mar 18. Blood. 2016. PMID: 26994149 Free PMC article.
References
-
- Brinkmann V. FTY720: mechanism of action and potential benefit in organ transplantation. Yonsei medical journal. [Review] 2004 Dec 31;45(6):991–997. - PubMed
-
- Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Current opinion in immunology. [Review] 2002 Oct;14(5):569–575. - PubMed
-
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacology & therapeutics. [Review] 2005 Dec;108(3):308–319. - PubMed
-
- Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. [Research Support, Non-U.S. Gov't Research Support US Gov't P.H.S. Review] 2001 Nov 30;294(5548):1875–1878. - PubMed
-
- Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacology & therapeutics. [Research Support, Non-U.S. Gov't Review] 2000 Nov;88(2):115–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

