Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal-weight and obese women
- PMID: 22727346
- PMCID: PMC3403702
- DOI: 10.1016/j.ajog.2012.04.022
Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal-weight and obese women
Erratum in
- Am J Obstet Gynecol. 2013 Apr;208(4):326
Abstract
Objective: Many observational studies indicate higher oral contraceptive failure among obese women, but most clinical trials and physiologic studies do not support these differences. Limited data indicate higher failure rates among obese contraceptive patch users. Data regarding contraceptive vaginal ring performance in obese women are needed.
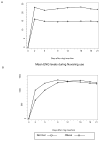
Study design: Twenty normal weight (body mass index [BMI] 19.0-24.9; median, 21.65) and 20 obese (BMI 30.0-39.9; median, 33.7) women enrolled in a prospective study of ethinyl estradiol (EE(2)) and etonorgestrel pharmacokinetics and of ovarian follicle development, endometrial thickness, and bleeding patterns, all measured biweekly during the second cycle of contraceptive vaginal ring use.
Results: Thirty-seven women completed follow-up. Mean day 0-21 EE(2) concentrations were lower among obese vs normal weight women (15.0 vs 22.0 pg/mL, respectively, P = .004), whereas etonorgestrel concentrations were similar (1138 vs 1256 pg/mL, respectively, P = .39). Follicular development was minimal in both groups, with only 5 women achieving a maximum follicle diameter >13 mm at any time during 3 weeks follow-up (3 normal weight and 2 obese women); these women had serum progesterone levels <1.0. Obese women reported more bleeding or spotting than normal weight women (3.6 vs 1.4 days, respectively, P = .01).
Conclusion: Although obese women had lower EE(2) levels during contraceptive vaginal ring use, they had excellent suppression of ovarian follicle development, similar to normal weight women. This predicts that contraceptive vaginal ring effectiveness will be similar in women with a BMI up to 39.9. The lower serum EE(2) levels in the obese women may explain the greater reported bleeding or spotting days.
Copyright © 2012 Mosby, Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST: C.L.W. is an advisory board member of Teva and Agile and a consultant for Merck and Bayer. The remaining authors report no conflict of interest.
Figures
Similar articles
-
Contraceptive vaginal ring effectiveness is maintained during 6 weeks of use: a prospective study of normal BMI and obese women.Contraception. 2013 Apr;87(4):432-6. doi: 10.1016/j.contraception.2012.12.001. Epub 2012 Dec 10. Contraception. 2013. PMID: 23312933 Free PMC article. Clinical Trial.
-
A comparative study of two contraceptive vaginal rings releasing norethindrone acetate and differing doses of ethinyl estradiol.Contraception. 1999 May;59(5):305-10. doi: 10.1016/s0010-7824(99)00036-0. Contraception. 1999. PMID: 10494484 Clinical Trial.
-
Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55-mg ethinyl estradiol/2.1-mg gestodene: a multicenter, open-label, uncontrolled study over three treatment cycles.Contraception. 2014 Sep;90(3):272-9. doi: 10.1016/j.contraception.2014.04.018. Epub 2014 May 20. Contraception. 2014. PMID: 24969733 Clinical Trial.
-
Contraceptive vaginal ring.Clin Obstet Gynecol. 2007 Dec;50(4):878-85. doi: 10.1097/GRF.0b013e318159c07e. Clin Obstet Gynecol. 2007. PMID: 17982330 Review.
-
Vaginal ring contraception.Contraception. 2011 Feb;83(2):107-15. doi: 10.1016/j.contraception.2010.07.008. Epub 2010 Oct 6. Contraception. 2011. PMID: 21237335 Review.
Cited by
-
Pharmacokinetics of Hormonal Contraception in Individuals with Obesity: a Review.Curr Obstet Gynecol Rep. 2020 Jun;9(2):72-78. doi: 10.1007/s13669-020-00284-y. Epub 2020 May 4. Curr Obstet Gynecol Rep. 2020. PMID: 33117601 Free PMC article.
-
Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration.Am J Obstet Gynecol. 2017 Jun;216(6):586.e1-586.e6. doi: 10.1016/j.ajog.2017.01.036. Epub 2017 Jan 29. Am J Obstet Gynecol. 2017. PMID: 28147241 Free PMC article.
-
Contraceptive Options Following Gestational Diabetes: Current Perspectives.Open Access J Contracept. 2019 Oct 22;10:41-53. doi: 10.2147/OAJC.S184821. eCollection 2019. Open Access J Contracept. 2019. PMID: 31749639 Free PMC article.
-
Obesity and hormonal contraceptive efficacy.Womens Health (Lond). 2013 Sep;9(5):453-66. doi: 10.2217/whe.13.41. Womens Health (Lond). 2013. PMID: 24007251 Free PMC article. Review.
-
Combined hormonal contraceptive (CHC) use among obese women and contraceptive effectiveness: a systematic review.Contraception. 2017 Feb;95(2):117-129. doi: 10.1016/j.contraception.2016.10.010. Epub 2016 Nov 4. Contraception. 2017. PMID: 27823942 Free PMC article. Review.
References
-
- Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820–7. - PubMed
-
- Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Darling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46–52. - PubMed
-
- Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002 Feb;77(2 Suppl 2):S13–8. - PubMed
-
- Dinger JC, Cronin M, Möhner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009 Sep;201(3):263. e1–9. - PubMed
-
- Dinger J, Minh TD, Buttmann N, Bardenheuer K. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol. 2011 Jan;117(1):33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical