Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases
- PMID: 22726684
- PMCID: PMC3383629
- DOI: 10.1016/j.chembiol.2012.05.009
Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases
Abstract
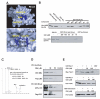
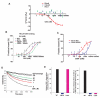
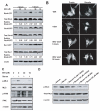
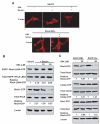
Rho GTPases have been implicated in diverse cellular functions and are potential therapeutic targets. By virtual screening, we have identified a Rho-specific inhibitor, Rhosin. Rhosin contains two aromatic rings tethered by a linker, and it binds to the surface area sandwiching Trp58 of RhoA with a submicromolar Kd and effectively inhibits GEF-catalyzed RhoA activation. In cells, Rhosin specifically inhibited RhoA activity and RhoA-mediated cellular function without affecting Cdc42 or Rac1 signaling activities. By suppressing RhoA or RhoC activity, Rhosin could inhibit mammary sphere formation by breast cancer cells, suppress invasion of mammary epithelial cells, and induce neurite outgrowth of PC12 cells in synergy with NGF. Thus, the rational designed RhoA subfamily-specific small molecule inhibitor is useful for studying the physiological and pathologic roles of Rho GTPase.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
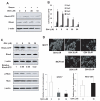
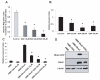
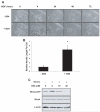
Similar articles
-
Rational design of Rho GTPase-targeting inhibitors.Methods Mol Biol. 2012;928:29-38. doi: 10.1007/978-1-62703-008-3_3. Methods Mol Biol. 2012. PMID: 22956131
-
Structure-function based design of small molecule inhibitors targeting Rho family GTPases.Curr Top Med Chem. 2006;6(11):1109-16. doi: 10.2174/156802606777812095. Curr Top Med Chem. 2006. PMID: 16842149 Review.
-
Approaches of targeting Rho GTPases in cancer drug discovery.Expert Opin Drug Discov. 2015;10(9):991-1010. doi: 10.1517/17460441.2015.1058775. Epub 2015 Jun 18. Expert Opin Drug Discov. 2015. PMID: 26087073 Free PMC article. Review.
-
RhoA inhibitor-eluting stent attenuates restenosis by inhibiting YAP signaling.J Vasc Surg. 2019 May;69(5):1581-1589.e1. doi: 10.1016/j.jvs.2018.04.073. J Vasc Surg. 2019. PMID: 31010523
-
Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3155-60. doi: 10.1073/pnas.1212324110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382194 Free PMC article.
Cited by
-
Loss of RhoA Exacerbates, Rather Than Dampens, Oncogenic K-Ras Induced Lung Adenoma Formation in Mice.PLoS One. 2015 Jun 1;10(6):e0127923. doi: 10.1371/journal.pone.0127923. eCollection 2015. PLoS One. 2015. PMID: 26030593 Free PMC article.
-
Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching.Br J Cancer. 2016 Jul 26;115(3):364-70. doi: 10.1038/bjc.2016.201. Epub 2016 Jun 30. Br J Cancer. 2016. PMID: 27362730 Free PMC article.
-
Synthesis and in vitro evaluation of benzo[b]thiophene-3-carboxylic acid 1,1-dioxide derivatives as anticancer agents targeting the RhoA/ROCK pathway.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2390911. doi: 10.1080/14756366.2024.2390911. Epub 2024 Sep 11. J Enzyme Inhib Med Chem. 2024. PMID: 39258708 Free PMC article.
-
Cdc42 regulates cytokine expression and trafficking in bronchial epithelial cells.Front Immunol. 2022 Dec 23;13:1069499. doi: 10.3389/fimmu.2022.1069499. eCollection 2022. Front Immunol. 2022. PMID: 36618374 Free PMC article.
-
Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma.Sci Rep. 2020 Mar 31;10(1):5712. doi: 10.1038/s41598-020-62690-9. Sci Rep. 2020. PMID: 32235878 Free PMC article.
References
-
- Akbar H, Cancelas J, Williams DA, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006;406:554–565. - PubMed
-
- Bellizzi A, Mangia A, Chiriatti A, Petroni S, Quaranta M, Schittulli F, Malfettone A, Cardone RA, Paradiso A, Reshkin SJ. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int. J. Mol. Med. 2008;22:25–31. - PubMed
-
- Boettner B, Van Aelst L. The role of Rho GTPases in disease development. Gene. 2002;286:155–174. - PubMed
-
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008;118:64–78. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

