Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease
- PMID: 22719003
- PMCID: PMC4571141
- DOI: 10.1093/brain/aws133
Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease
Abstract
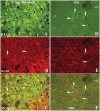
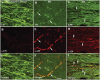
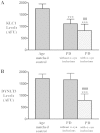
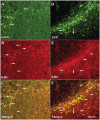
The progressive loss of the nigrostriatal pathway is a distinguishing feature of Parkinson's disease. As terminal field loss seems to precede cell body loss, we tested whether alterations of axonal transport motor proteins would be early features in Parkinson's disease. There was a decline in axonal transport motor proteins in sporadic Parkinson's disease that preceded other well-known nigral cell-related pathology such as phenotypic downregulation of dopamine. Reductions in conventional kinesin levels precede the alterations in dopaminergic phenotypic markers (tyrosine hydroxylase) in the early stages of Parkinson's disease. This reduction was significantly greater in nigral neurons containing α-synuclein inclusions. Unlike conventional kinesin, reductions in the levels of the cytoplasmic dynein light chain Tctex type 3 subunit were only observed at late Parkinson's disease stages. Reductions in levels of conventional kinesin and cytoplasmic dynein subunits were recapitulated in a rat genetic Parkinson's disease model based on over-expression of human mutant α-synuclein (A30P). Together, our data suggest that α-synuclein aggregation is a key feature associated with reductions of axonal transport motor proteins in Parkinson's disease and support the hypothesis that dopaminergic neurodegeneration following a 'dying-back' pattern involving axonal transport disruption.
Figures
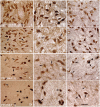
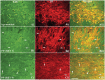
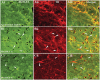
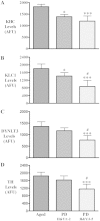
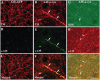
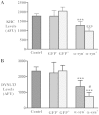
Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Alterations in Activity-Dependent Neuroprotective Protein in Sporadic and Experimental Parkinson's Disease.J Parkinsons Dis. 2016;6(1):77-97. doi: 10.3233/JPD-160812. J Parkinsons Dis. 2016. PMID: 27003787
-
Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson's disease model.Acta Neuropathol. 2019 Oct;138(4):575-595. doi: 10.1007/s00401-019-02023-x. Epub 2019 May 31. Acta Neuropathol. 2019. PMID: 31165254 Free PMC article.
-
Number of kinesins engaged in axonal cargo transport: A novel biomarker for neurological disorders.Neurosci Res. 2023 Dec;197:25-30. doi: 10.1016/j.neures.2023.09.004. Epub 2023 Sep 19. Neurosci Res. 2023. PMID: 37734449 Review.
-
Axonal transport and neurodegenerative disease: can we see the elephant?Prog Neurobiol. 2012 Dec;99(3):186-90. doi: 10.1016/j.pneurobio.2012.03.006. Epub 2012 Apr 1. Prog Neurobiol. 2012. PMID: 22484448 Free PMC article. Review.
Cited by
-
Nanoparticles in the brain: a potential therapeutic system targeted to an early defect observed in many neurodegenerative diseases.Pharm Res. 2013 Oct;30(10):2459-74. doi: 10.1007/s11095-013-1037-0. Epub 2013 Apr 27. Pharm Res. 2013. PMID: 23625095 Review.
-
Microtubule Destabilization Paves the Way to Parkinson's Disease.Mol Neurobiol. 2017 Nov;54(9):6762-6774. doi: 10.1007/s12035-016-0188-5. Epub 2016 Oct 18. Mol Neurobiol. 2017. PMID: 27757833 Review.
-
The prion hypothesis of Parkinson's disease.Curr Neurol Neurosci Rep. 2015 May;15(5):28. doi: 10.1007/s11910-015-0549-x. Curr Neurol Neurosci Rep. 2015. PMID: 25868519
-
Decreased Exosomal Acetylcholinesterase Activity in the Plasma of Patients With Parkinson's Disease.Front Aging Neurosci. 2021 May 28;13:665400. doi: 10.3389/fnagi.2021.665400. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34122043 Free PMC article.
-
BDNF-Regulated Modulation of Striatal Circuits and Implications for Parkinson's Disease and Dystonia.Biomedicines. 2024 Aug 5;12(8):1761. doi: 10.3390/biomedicines12081761. Biomedicines. 2024. PMID: 39200225 Free PMC article. Review.
References
-
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–49. - PubMed
-
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–98. - PubMed
-
- Chu Y, Mickiewicz AL, Kordower JH. α-Synuclein aggregation reduces nigral myocyte enhancer factor-2D in idiopathic and experimental Parkinson’s disease. Neurobiol Dis. 2011;41:71–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

